As health officials brace for a challenging respiratory illness season, marked by the resurgence of influenza and the ongoing threat of COVID-19, they are urging the public to receive flu shots and reformulated COVID boosters. However, protection against respiratory syncytial virus (RSV)-related lung disease is currently unavailable. Addressing this gap, a recent study has shed light on the crucial role of human T cells in controlling RSV infection, opening new avenues for vaccine development.
RSV is a highly contagious respiratory virus that typically causes mild cold symptoms in healthy adults. However, in infants, the immunocompromised, and older individuals, it can lead to severe lung infections.
Previous vaccine efforts against RSV have primarily focused on generating an antibody response. However, utilizing innovative precision animal models of RSV infection, researchers have gained fresh insights into how the human immune system, particularly T cells, can effectively control and eliminate RSV (Figure 1). The study demonstrated that T cells can independently combat RSV infection in human lung tissue, even in the absence of RSV-specific antibodies. While a vaccine-induced RSV-specific T cell response may not prevent infection, it can accelerate viral clearance and mitigate disease severity when antibody-based protection fails due to antigenic variability among circulating strains.
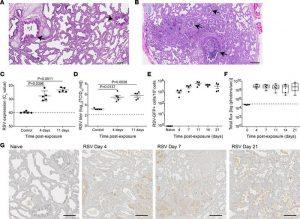
Figure 1: Sustained replication of RSV in human lung implants. (A and B) H&E staining of (A) a naive LoM human lung implant (n = 5 implants analyzed) and (B) a human lung implant from an RSV-infected LoM (n = 12 implants analyzed). Scale bars: 200 μm. Arrows note airways. (C) RSV-RNA expression in the human lung implants of control (2 hours after RSV exposure) and RSV-infected LoM at 4 and 11 days after exposure (n = 5 implants/time point). Crossing point (Cp) indicates the cycle number at which the fluorescence signal of the sample exceeds a background fluorescence value. (D) RSV titers (log10TCID50/mL) in human lung implants of control LoM (2 hours after RSV exposure) and RSV-infected LoM at 4 days and 11 days after RSV exposure (n = 5 implants/time point). Dashed line indicates the assay limit of detection. (E) Number of GFP+ cells as determined by flow cytometry in the human lung implants of naive LoM (n = 4 implants) and RSV A2-GFP–infected LoM 4, 7, 11, 14, and 21 days after exposure (n = 4 implants/time point). (F) RSV replication monitored longitudinally in the human lung implants of RSV-Luc infected LoM (n = 6 implants) as measured by bioluminescence (radiance [p sec–1 cm–2 sr–1] represented as total flux) signal. The median (horizontal line), upper and lower quartiles (box ends), and minimum to maximum values (whiskers) are shown. Background luminescence (day 0) is denoted by the dashed line. (G) IHC staining for RSV antigen in the human lung implants of naive LoM (n = 4 implants analyzed) and RSV-infected LoM 4, 7, and 21 days after exposure (positive cells are brown, n = 4 implants analyzed/time point). Scale bars: 200 μm. (C–E) Data are shown as the mean ± SEM; (C and D) a statistical analysis was performed using a 2-tailed Kruskal-Wallis test. P values were adjusted for multiple testing using the Benjamini, Krieger, and Yekutieli FDR method.
Developing an effective and safe RSV vaccine is a top priority. However, the incomplete understanding of how the human immune response combats RSV infection has posed a significant obstacle. This study’s findings provide a promising pathway for future vaccine development by highlighting the pivotal role of T cells in controlling RSV and the potential for enhancing immune responses against the virus.
Journal article: De, C., et al., 2023. Human T cells efficiently control RSV infection. JCI Insight.
Summary by Stefan Botha










