Diagnostic Tests
 This section has been designed to assist you with understanding the use of the diagnostic tests used in immunological testing and clinical practice.
This section has been designed to assist you with understanding the use of the diagnostic tests used in immunological testing and clinical practice.
If you would like to have a specific diagnostic test featured please Contact Us.
- Alere HIV Combo - Rapid Test
- ELISA tests
- Lymphocyte Proliferation Test
- PCR test
- Mantoux test
- Viral Load Assays
- Flow Cytometry
- Hepatitis B Surface Antigen
- Rapid HIV Test
- Ouchterlony test
- CD4 Count
Alere™ HIV Combo – Rapid Test
- in vitro
- Qualitative immunoassay
- 4th generation Rapid Test
- Detection of antibodies (Ab) to HIV-1 and HIV-2
- Detection of free HIV-1 p24 antigen (Ag)
- As it detects p24 antigens, it may identify HIV infection earlier than antibody-only tests
The multiplication of the HIV in the infected cells leads to cell rupture and thus the release of HIV virus particles, which are first detected in the form of HIV RNA and next in the form of HIV antigen. This is followed by production of specific antibodies to either HIV-1 or HIV-2. The HIV antigen may become undetectable at this time because of the formation of antibody-antigen complexes. Alere HIV Combo can only detect free p24 antigens.
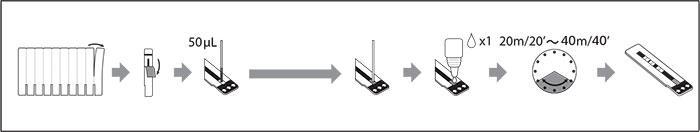
Test Procedure
- Rapid and easy-to-use
- Lateral flow assay
- 50 μL of sample applied to a sample pad
- 1 drop of Chaser Buffer applied to sample pad
- Sample diffuses along the strip
- Results for HIV-1 p24 antigen and HIV-1 and HIV-2 antibodies
- Control window
- Results read in designated windows
- The small strip format is suitable for use with whole blood, serum, or plasma samples
- Results in 20-40 minutes
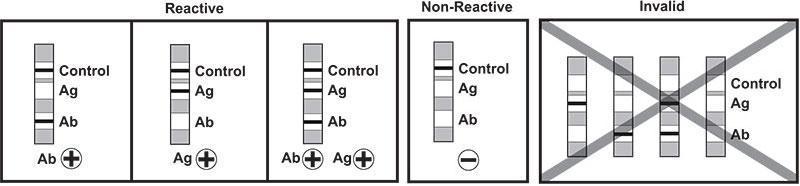
Test Results
- Control and Ab bars – Antibody reactive
- Control and Ag bars – Antigen p24 reactive
- Control, Ab and Ag bars – Antibody and Antigen p24 reactive
- Only Control bar – Non-reactive
- No Control bar – Test invalid
How the Test Works
- A specimen is added to the sample pad.
- The specimen mixes with biotinylated anti-p24 antibodies and selenium colloid-conjugates coated with recombinant HIV-1, HIV-2 and HIV-1 group O antigens, synthetic HIV-2 peptide and anti p24 mouse monoclonal antibody.
- This mixture continues to migrate through the solid phase to the immobilized recombinant HIV-1/HIV-1 group O antigens and synthetic HIV-1/HIV-2 peptides at the Antibody (Ab) window, immobilized avidin at the Antigen (Ag) window.
- If antibodies to HIV-1 and/or HIV-2 are present in the specimen, the antibodies bind to the selenium colloid-conjugates coated with recombinant HIV-1, HIV-2 and HIV-1 group O antigens and synthetic HIV-2 peptide and to the immobilized recombinant HIV-1/HIV-1 group O antigens and synthetic HIV-1/HIV-2 peptides, forming one red bar at the Ab window site.
- If antibodies to HIV-1 and HIV-2 are absent, the selenium colloid-conjugates flow past the Ab window and no red bar is formed at the Ab window site.
- If free HIV-1 p24 antigen is present in the specimen, the antigen binds to the biotinylated anti-p24 antibodies and the selenium colloid-conjugate coated with anti p24 mouse monoclonal antibody. This complex binds to the immobilized avidin forming a red bar at the Ag window site.
- If HIV-1 p24 antigen is not present, both the biotinylated anti-p24 antibodies and selenium colloid conjugate flow past the Ag window and no red bar is formed at the Ag window site.
- To ensure assay validity, a procedural control bar is incorporated in the assay device at the Control window.
Test Limitations
- No test provides absolute assurance that a specimen does not contain low levels of HIV-1 p24 antigen and/or
antibodies to HIV-1 and HIV-2 such as those present at a very early stage or late stage of HIV infection. - Alere HIV Combo is designed to detect antibodies to HIV-1 and/or HIV-2 and free HIV-1 p24 antigen, in human serum, plasma and capillary and venous whole blood specimens. Other body fluids or pooled specimens should not be used.
- The intensity of the Ab and Ag bars does not necessarily correlate to the titer of antibody and antigen in the specimen.
- The absence of Ag bar may occur when all p24 antigen is bound by antibodies. When high levels of antibodies against the p24 antigen are present in the blood after seroconversion, the antibodies tend to bind to the antigens, forming immunocomplexes. Alere HIV Combo detects only non-immunocomplexed (free) antigens; it does not detect immunocomplexed (bound) antigens.
- HIV-infected persons taking antiretroviral medication have been shown to produce false negative results when tested by rapid diagnostic tests.
- Infants born to HIV-infected mothers may carry maternal antibodies and will test antibody positive until eighteen months of age, which may not necessarily indicate the true infection status of the new born. The use of HIV- 1 p24 antigen testing to exclude infection in neonates (up to around eighteen months) is not recommended by CDC, because of poor sensitivity, especially in the presence of HIV antibody. Definitive diagnosis of HIV infection in early infancy requires other assays, including HIV nucleic acid test or viral culture.
More Information
Alere.com – Alere HIV Combo
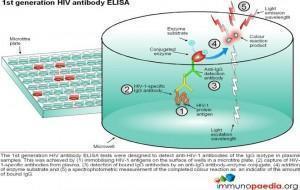
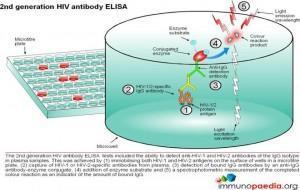
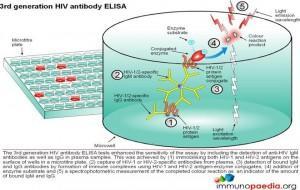
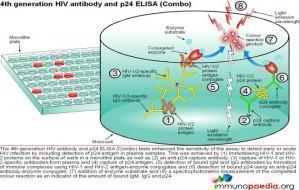
ELISA tests
ELISA (enzyme –linked immunosorbent assay) is a serological assay in which a bound antigen or antibody is detected by a linked enzyme that converts a colourless substrate into a coloured product. There are 4 generations of HIV antibody ELISA tests. The progression of these assays has been depicted in our series of 4 graphics.
Download ELISA Tests Images
Lymphocyte Proliferation Test
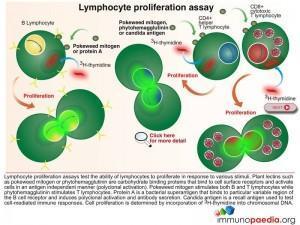 The Lymphocyte Proliferation Assay (LPA) is a test used to measure the ability of lymphocytes to proliferate in response to various stimuli such as candida, pokeweed and phytohaemagglutinin. These plant lectins are carbohydrate binding proteins that bind to cell surface receptors and activate cells in an antigen independent manner. Most people will respond to at least one of several common microbial antigens. LPA can be used to measure improvements in immunological function following antiretroviral therapy and to detect the presence of immune responses against specific opportunistic pathogens.
The Lymphocyte Proliferation Assay (LPA) is a test used to measure the ability of lymphocytes to proliferate in response to various stimuli such as candida, pokeweed and phytohaemagglutinin. These plant lectins are carbohydrate binding proteins that bind to cell surface receptors and activate cells in an antigen independent manner. Most people will respond to at least one of several common microbial antigens. LPA can be used to measure improvements in immunological function following antiretroviral therapy and to detect the presence of immune responses against specific opportunistic pathogens.
The 3H-thymidine incorporation assay is used to determine the extent of cell division in response to a proliferation signal. 3H-thymidine which is a radioactive nucleoside and precursor of thymine found in DNA becomes incorporated in DNA strands of proliferating cells and can be measured to determine extent of cell proliferation.
Download Lymphocyte Proliferation Assay Images
PCR test
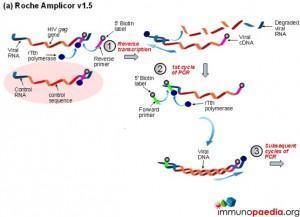
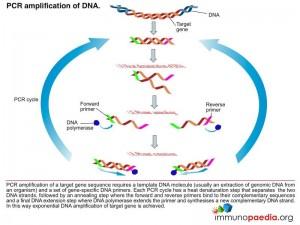 The polymerase chain reaction (PCR) is a technique used for a wide range of tasks including detection and diagnosis of infectious disease. The technique exponentially amplifies a fragment of DNA or RNA by in vitro enzymatic replication and because PCR amplifies the regions of DNA or RNA that it targets, PCR can be used to analyze extremely small amounts of sample. Viral DNA or RNA can therefore be detected by PCR. The primers used need to be specific to the targeted sequences in the DNA or RNA of a virus, and the PCR can be used for diagnostic analyses or DNA or RNA sequencing of the viral genome. The high sensitivity of PCR permits virus detection soon after infection and even before the onset of disease, allowing for a significant lead in treatment. A patient’s viral load can also be quantified by PCR-based DNA or RNA quantitation techniques.
The polymerase chain reaction (PCR) is a technique used for a wide range of tasks including detection and diagnosis of infectious disease. The technique exponentially amplifies a fragment of DNA or RNA by in vitro enzymatic replication and because PCR amplifies the regions of DNA or RNA that it targets, PCR can be used to analyze extremely small amounts of sample. Viral DNA or RNA can therefore be detected by PCR. The primers used need to be specific to the targeted sequences in the DNA or RNA of a virus, and the PCR can be used for diagnostic analyses or DNA or RNA sequencing of the viral genome. The high sensitivity of PCR permits virus detection soon after infection and even before the onset of disease, allowing for a significant lead in treatment. A patient’s viral load can also be quantified by PCR-based DNA or RNA quantitation techniques.
Download PCR Test Images
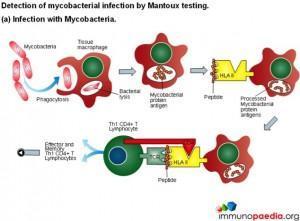
Mantoux test
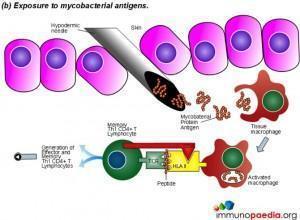
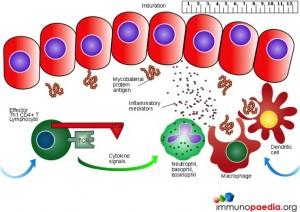
The Mantoux test also called the Purified Protein Derivative (PPD test) or Tuberculin Sensitivity Test is a diagnostic tool for tuberculosis. The test shows if a person has been exposed to TB bacteria, but it does not indicate if the infection is active or latent or if the patient is currently infectious. This test is an example of a Type IV or cell-mediated/delayed hypersensitivity immune response.
Download Mantoux Test Images
Viral Load Assays
The viral load assay is used to monitor the status of HIV disease, in conjunction with the CD4 count and physical disease progression. It is also used to guide therapy and to predict the progression of HIV. A viral load is conducted when a patient is first diagnosed with HIV, at the start of therapy or when a therapy is changed and regularly thereafter to monitor any changes. With effective therapy, viral loads typically decrease within 3-6 months. When a patient has <25 copies of virus they are said to be virally suppressed. Evidence shows that keeping the viral load levels as low as possible for as long as possible decreases the complications of HIV disease.
There are several methods for testing viral load; results are not interchangeable so it is important that the same method be used each time.
The 5 methods for testing Viral Load are:
a) Roche Amplicor
b) Versant Branched DNA
c) Roche COBAS taqman
d) Nuclisens
e) Abbot HIV real-time quantification
Download Viral Load Assays Images
Flow Cytometry
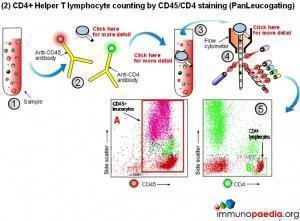
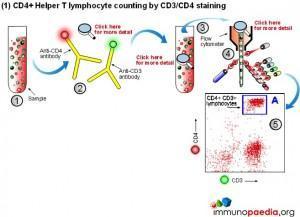 Manual methods using cell selection and microscope counting techniques can be used, however, automated counting of CD4+ Helper T lymphocytes in blood is usually performed on a flow cytometer. Although more expensive, this method is more accurate and less labour intensive.
Manual methods using cell selection and microscope counting techniques can be used, however, automated counting of CD4+ Helper T lymphocytes in blood is usually performed on a flow cytometer. Although more expensive, this method is more accurate and less labour intensive.
The flow cytometer uses fluorescent cell surface markers (usually these are labelled antibodies) to identify the subset of CD4+ Helper T lymphocytes present in blood samples.
There are two approaches regarding the type of cell surface markers used.
1) Conventional CD4 counting (CD3 and CD4 labelling)
The conventional method makes use of fluorescent antibodies to CD3 and CD4 cell surface receptors. CD3 is part of the T cell receptor (TCR) and is expressed on CD8+ Cytotoxic T lymphocytes and CD4+ Helper T lymphocytes. CD4 is expressed on Helper T lymphocytes and monocytes. Using fluorescence detection on a flow cytometer the fraction of total lymphocytes double-positive for CD3 and CD4 (i.e. CD4+ Helper lymphocytes) can be measured. For single platform assays the absolute number of the double-positive cells can be inferred from calibrated bead standards or by determining the volume of blood used with a volumetric flow cytometer. The dual-platform method relies on a full blood count by a haematology analyser to provide a value for the total lymphocyte count, while the flow cytometer gives a percentage of lymphocytes that are CD4+ Helper T lymphocytes. Together these readings can be used to calculate absolute CD4 counts. The most reliable and robust counting procedure is the single platform assay.
2) CD4 counting by the PanLeucogate method (CD45 and CD4 labelling)
An alternative labelling method uses fluorescent antibodies to CD45 and CD4 cell surface receptors. CD45 is expressed on all leucocytes and is not expressed on mature red blood cells (erythrocytes) or platelets (thrombocytes). It therefore serves as a marker of total white blood cells. CD4 is expressed on Helper T lymphocytes and monocytes. The dual platform method requires a haematology analyzer to provide only the total leukocyte count. This is the reason PanLeucogating is preferred when using the dual platform method, only a total white cell count is needed from the haematology analyzer and the need for a differential count (the lymphocyte count) from the haematology analyser is no longer necessary. The leucocytes serve as a common counting parameter when using the flow cytometer and haematology analyzer. Since CD4 is expressed on both Helper T lymphocytes and monocytes the light scatter parameters available on the flow cytometer are used to differentiate between them because of differences in complexity between the populations. This method can also be used on a single platform basis using calibrated bead standards or a volumetric flow cytometer to calculate the absolute CD4 count.
Hepatitis B Surface Antigen
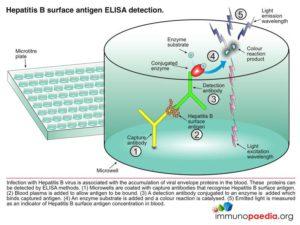 Hepatitis B surface antigen (HBsAg) is a protein antigen produced by hepatitis B virus (HBV). This antigen is the earliest indicator of acute hepatitis B infection and is able to identify infected people before they are symptomatic. HBsAg becomes undetectable in the blood during the recovery period. Children and immunocompromised patients such as patients with HIV infection who become chronically infected may remain HBsAg positive.
Hepatitis B surface antigen (HBsAg) is a protein antigen produced by hepatitis B virus (HBV). This antigen is the earliest indicator of acute hepatitis B infection and is able to identify infected people before they are symptomatic. HBsAg becomes undetectable in the blood during the recovery period. Children and immunocompromised patients such as patients with HIV infection who become chronically infected may remain HBsAg positive.
To detect this antigen we perform an Enzyme-Linked Immunosorbent Assay test (HIV ELISA).
Download HBV ELISA Images
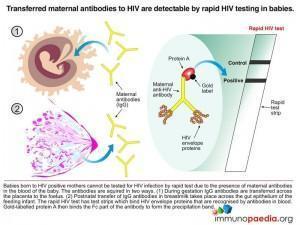 A baby born to a sero-positive mother will also be sero-positive. This positivity can last up to the first 18 months of life because the test detects maternal antibodies. For this reason, it is useless to test the baby at two months using an HIV antibody test as IgG will have either crossed the placenta to the baby in utero or via beast milk postnatal. Until these antibodies disappear the baby will continue to test sero-positive. After 18 months, if the baby is truly infected with HIV, the child’s own immune system will be the cause of a sero-positive antibody test.
A baby born to a sero-positive mother will also be sero-positive. This positivity can last up to the first 18 months of life because the test detects maternal antibodies. For this reason, it is useless to test the baby at two months using an HIV antibody test as IgG will have either crossed the placenta to the baby in utero or via beast milk postnatal. Until these antibodies disappear the baby will continue to test sero-positive. After 18 months, if the baby is truly infected with HIV, the child’s own immune system will be the cause of a sero-positive antibody test.
Download Rapid HIV Test – Infants Images
Ouchterlony test
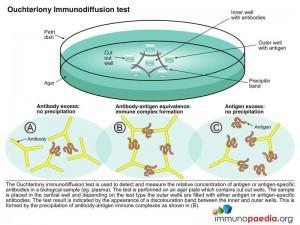
Immune precipitates can form in an agar matrix. When antigen and antibody diffuse toward one another in agar, or when antibody is incorporated into the agar and antigen diffuses into the antibody containing matrix, a visible line of precipitation will form. As in a precipitation reaction in fluid, visible precipitation occurs in the region of equivalence, whereas no visible precipitate forms in regions of antibody or antigen excess. This immunodiffusion reaction can be used to determine relative concentrations of antibodies or antigens, to compare antigens or to determine the relative purity of an antigen preparation.
In the Ouchterlony method both antigen and antibody diffuse radially from wells toward each other, thereby establishing a concentration gradient. As equivalence is reached, a visible line of precipitation forms. This is a simple and effective qualitative tool for determining the relationship between antigens and the number of different Ag-Ab systems present.
Download Ouchterlony Test Images
CD4 Count
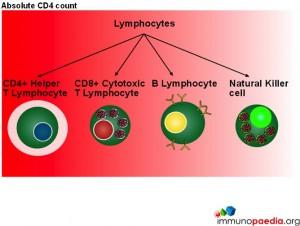 In this series of graphics the three basic cell types found in blood (leukocytes, erythrocytes and platelets) have been depicted. The graphics further explain the leukocyte cell lines and include the granulocytes, monocytes and lymphocytes and the different cells which make up these groups of cells.
In this series of graphics the three basic cell types found in blood (leukocytes, erythrocytes and platelets) have been depicted. The graphics further explain the leukocyte cell lines and include the granulocytes, monocytes and lymphocytes and the different cells which make up these groups of cells.
When measuring disease states it is an absolute CD4 count which is used to monitor patients.
Although CD4 cell counts are used to measure HIV disease states absolute CD4 counts are not a reliable marker when determining disease states in children. 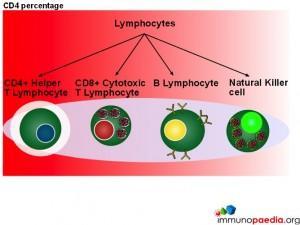 This is because lymphocytes are present in higher numbers in children thus making the count less accurate. The WHO has therefore recommended that all children under the age of 5 should be evaluated with a CD4 percentage when assessing disease status and decision making for initiation of treatment.
This is because lymphocytes are present in higher numbers in children thus making the count less accurate. The WHO has therefore recommended that all children under the age of 5 should be evaluated with a CD4 percentage when assessing disease status and decision making for initiation of treatment.