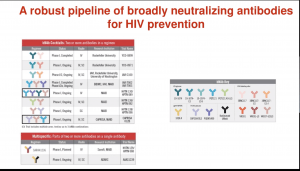
In the previous summary (IUIS Webinar: HIV prevention- antibodies and vaccine development part 1) we highlighted research presented by Professor Lynn Morris on the current state of HIV vaccines. In this summary, we highlight findings from the Antibody-Mediated Prevention (AMP) trial, a proof-of-concept trial to determine if passive immunisation could prevent HIV.
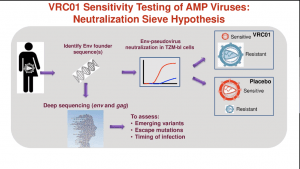 Prof Lynn Morris discussed results from the AMP trial that tested the ability of VRC01 bNAb that binds the HIV envelope protein CD4 binding site, has a wide coverage of HIV subtype B and C strains in vitro studies. Participants in the AMP trials received 10 administrations of VRC01 at 8-week intervals for 20-months because VRC01 has a short half-life (12 days). Unfortunately, the administration of VRC01 did not significantly lower the incidence of HIV acquisition. Analysis of HIV viral isolates from breakthrough infections demonstrated some efficacy (75%) against VRC01-sensitive HIV strains, which constituted a minority of isolates and was associated with a much lower viral load at onset of infection than individuals infected with VRC01 resistant strains. (read more: Does serial administration of HIV-specific VRC01 bnAbs prevent HIV acquisition?).
Prof Lynn Morris discussed results from the AMP trial that tested the ability of VRC01 bNAb that binds the HIV envelope protein CD4 binding site, has a wide coverage of HIV subtype B and C strains in vitro studies. Participants in the AMP trials received 10 administrations of VRC01 at 8-week intervals for 20-months because VRC01 has a short half-life (12 days). Unfortunately, the administration of VRC01 did not significantly lower the incidence of HIV acquisition. Analysis of HIV viral isolates from breakthrough infections demonstrated some efficacy (75%) against VRC01-sensitive HIV strains, which constituted a minority of isolates and was associated with a much lower viral load at onset of infection than individuals infected with VRC01 resistant strains. (read more: Does serial administration of HIV-specific VRC01 bnAbs prevent HIV acquisition?).
Despite not showing efficacy, the trial did demonstrate that AMP is feasible. Prof Morris also highlighted that other bNAbs have been found that have a 10-50 fold higher neutralisation capacity than VRC01 and are currently being tested in pre-clinical and clinical studies. Prof Morris then highlighted an ongoing clinical phase I trial (CAP012) that is testing CAP256-CRC26.25 bNAb highly effective against clade C HIV viruses, alone or in combination with another Ab (VRC07-523LS), and has been shown to protect NHPs. Thus far, the passive immunisation is safe, and in vivo sera levels last at least 16 weeks, phase II trial will begin later this year.
Summary by Cheleka AM Mpande