- Patient presentation
- History
- Differential diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final Outcome
- References
- Evaluation - Questions & answers
- MCQs
Patient Presentation
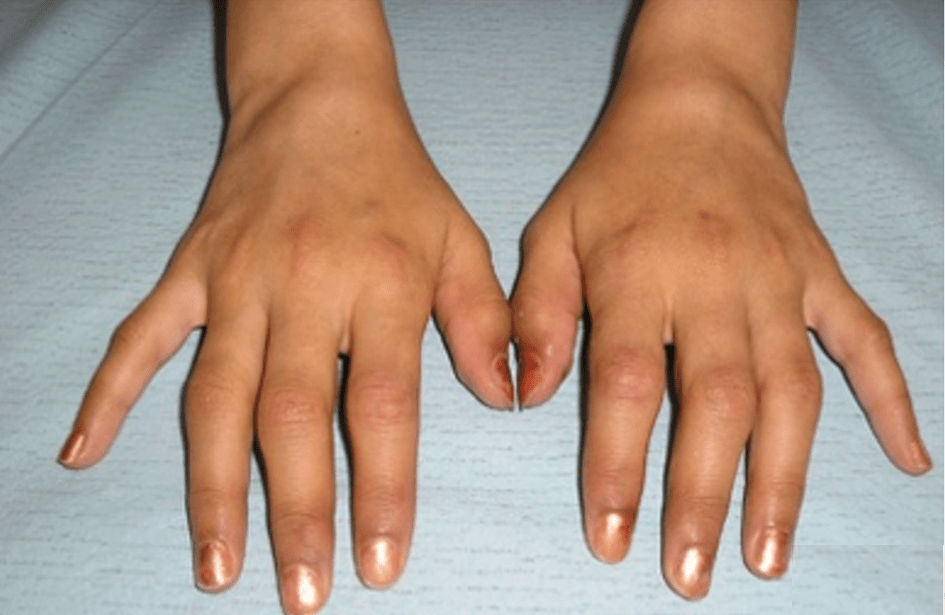
Eva is a 50-year-old Caucasian woman who presents to the clinic with complaints of hand and feet swelling to the point of visible deformities. Her joint pain wakes her up several times during the night and she has morning stiffness for several hours. She is a secretary and has noticed that her typing speed has decreased.
Acknowledgement
This case study was provided by Pr. Olivier Boyer (M.D., Ph.D., Head of the Department of Immunology and Biotherapy, Rouen University Hospital, France) and Dr. Pauline Brevet (M.D., Assistant Professor in Rheumatology, Rouen University Hospital, France) of the Faculty of Medicine of Rouen, Normandy University, France. The authors would like to thank Pr. Olivier Vittecoq (M.D., PhD, Department of Rheumatology, Rouen University Hospital, France), Dr Fabienne Jouen (M.D., Department of Immunology and Biotherapy, Rouen University Hospital, France), Pr. Elena Hsieh (Department of Allergy and Immunology, University of Colorado Anschutz School of Medicine, Aurora, USA) and Pr. Jane Buckner (Director of Benaroya Research Institute, Department of Immunology, Seattle, USA) for their critical reading.
History
The symptoms started 6 months ago. She referred to her general practitioner to complain about swelling and pain of the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints of both hands and the metatarsophalangeal (MTP) joints of both feet symmetrically. During the consultation, the pain was rated at 6 on the Visual Analogic Scale (VAS) which scores from 0 (no pain) to 10 (maximum pain). She did not complain of any other symptoms. She was prescribed oral corticosteroids at a dosage of 15 mg/day for 15 days, which improved her symptoms. However, the symptoms recurred after the end of the treatment.
Past medical history
Tobacco (cigarette smoker for 28 years) and current smoker.
Chronic gingivitis.
No allergies
Surgical history
Ligamentoplasty of the right knee ten years ago.
Family history
Her aunt suffered from rheumatoid arthritis.
Travel history
She travelled to Spain during her last holidays.
Social history
Secretary. 2 children and 3 grandchildren with no disease.
Medication
Corticosteroids 15mg/day for 15 days.
Differential diagnosis
- Connective Tissue Diseases (systemic lupus erythematosus, Sjögren syndrome, systemic sclerosis)
- Infectious polyarthritis (bacterial or viral)
- Acute rheumatic fever
- Metabolic arthropathies (chondrocalcinosis, gout)
- RS3PE syndrome (remitting seronegative symmetrical synovitis with pitting oedema)
- Osteoarthritis
- Paraneoplastic rheumatism
- Polymyalgia rheumatica
- Psoriatic rheumatism
- Auto-inflammatory disease with joint expression
- Vasculitis
- Amyloidosis
- Whipple’s disease
Examination
Vitals
- Heart rate: 60/min.
- Blood pressure: 124/82 mmHg.
- Temperature: 37.8°C.
- Oxygen saturation: 98%.
General
- She looks tired.
- No pallor.
- No purpura.
- No oedema of the lower limbs.
Cardiovascular
- Normal heart sounds.
- All pulses present.
Respiratory
- No dyspnea, respiratory rate 15/min.
- No chest deformity.
- Good bilateral air entry.
- No crackling sound on the lungs or wheezing.
Abdomen
- Normal.
Neurological
- No paralysis of other cranial nerves.
- Normal pupillary reflexes.
- Normal reflexes on upper and lower limbs.
Musculoskeletal system
- Normal power of lower and upper limbs.
- No back pain.
- No foot/toe pain.
Skin and ORL
- No skin or ORL disorder.
- No Raynaud syndrome.
Investigations
| Examination | Value | Normal limits |
|---|---|---|
| White blood cell | 12 | (4-12 x 109 /L) |
| Hemoglobin | 10.9 | (12.1-15.2 g / L) |
| VGM | 70 | (80-100 fL) |
| Ferritin | 450 | 10-270 umol / L |
| Platelets | 500 | (140-450 x 109/ L) |
| C-reactive protein | 50 | (0-8 mg/ l) |
| Sodium | 136 | (135-147 mmol/ L) |
| Potassium | 4.2 | (3.3-5.0 mmol/ L) |
| Urea | 6.2 | (2.5-6.4 mmol/ L) |
| Creatinine | 96 | (62-115 mmol/ L) |
| Creatinine kinase | 183 | (25-195 IU/ L) |
| Total protein | 62 | (60-80 g/ L) |
| Albumin | 37 | (35-50 g/ L) |
| Corrected calcium | 2.35 | (2.1-2.6 mmol /L) |
| Phosphate | 1.3 | (1.0-1.5 mmol /L) |
| Magnesium | 1.1 | (0.8-1.3 mmol /L) |
| Aspartate aminotransferase | 35 | (10-35 IU/L) |
| Alanine aminotransferase | 35 | (10-35 IU /L) |
| G-glutamyl transferase | 38 | (10-38 IU /L) |
| Alkaline phosphatase | 90 | (35-105 IU /L) |
| Bilirubin | 10 | (2-18 umol /L) |
| Prothrombin time | 85 | (75-100 %) |
| Thyroid stimulating hormone | 11.3 | (9-30 mIU /L) |
| Antinuclear antibodies | Negative | |
| Anti-neutrophil cytoplasmic antibodies | Negative | |
| (anti-myeloperoxydase (MPO) and anti-proteinase 3 - PR3 | ||
| Anti-citrullinated peptides antibodies | 900 | ≺ 7AU /mL |
| Rheumatoid factor | 459 | ≺ 20 IU /mL |
| Uric acid | 223 | ≺ 360 micromol /L |
| Proteinuria | 0.09 | ≺ 0.15 g/ 24h |
| HIV | Negative | |
| HBs antigen | Negative | |
| Anti-HBs antibodies | Positive | |
| Anti-HBc antibodies | Negative | |
| Anti-HCV antibodies | Negative |
Table 1. Biological data
Serum protein electrophoresis: Hyper a1 and a2 globulins, polyclonal g globulins increase.
EKG: normal.
Chest X-ray: normal.
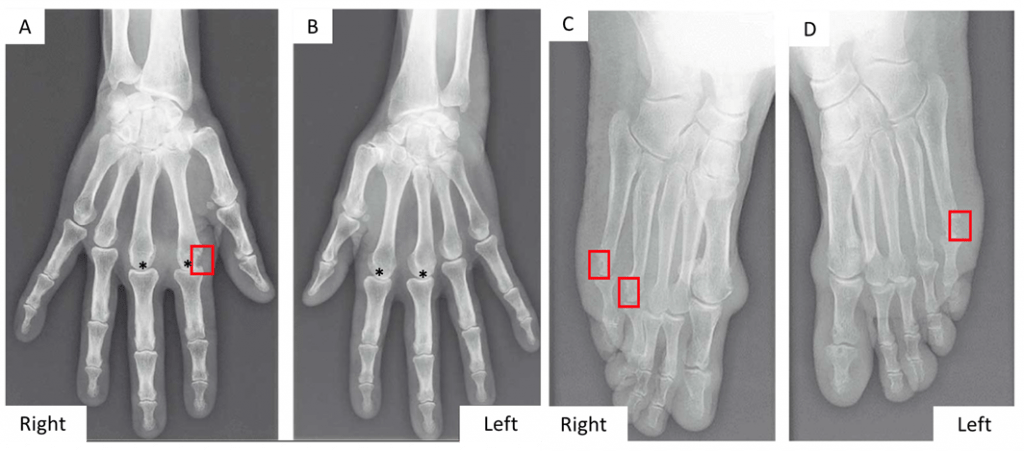
Hand and feet X-Rays: presence of an erosion of the right MCP2 (A, red square), the right MTP4 and MTP5 (C, red square), the left MTP5 (D, red square) and bilateral narrowing of several MCP2 and MCP3 (*) (Figure 2).
Follow-up:
Six months after symptom onset, she consulted with a rheumatologist who prescribed the investigations indicated above. Treatment was started with methotrexate at 10 mg then 15 mg orally once a week. The disease activity score (DAS28-CRP) is 3.4 (Table 2).
After 6 months, the DAS28-CRP was still at 3.4, which indicated insufficient therapeutic response. It was therefore decided to add subcutaneous anti-TNF therapy (adalimumab 40 mg) every 2 weeks. This therapy permitted the patient to undergo complete remission for 3 years.
Six months ago, she suffered a relapse and consulted her rheumatologist. Compliance with adalimumab was good. An anti-drug antibody assay was performed and found anti-adalimumab antibodies.
| DAS-28 Value | Clinical State | |||
|---|---|---|---|---|
| ≻ 2.6 | Remission | |||
| 2.6 to ≼ 3.2 | Low disease activity | |||
| 3.2 to ≼ 5.1 | Moderate disease activity | |||
| ≻ 5.1 | High disease activity |
Table 2: Disease activity score value of 28 joints with clinical significance
Discussion
The cause of the presenting condition is rheumatoid arthritis (RA).
Clinical picture and diagnostic criteria:
RA is the most common chronic inflammatory rheumatic disease, with a prevalence ranging from 0.5% to 1% in Europe and America, and around 0.3% in Africa and Asia. RA causes progressive disability and systemic complications. RA can occur at any age, including juvenile patients, with the greatest frequency near the age of 50. It affects women 3 times more frequently than men. Many patients still fail to get complete relief and the life expectancy of RA patients is still decreased despite current therapies. The diagnosis of RA is defined according to the 2010 American College of Rheumatology and European League Against Rheumatism (ACR/EULAR) classification criteria (Table 3).
| Domain | Category | Point score |
|---|---|---|
| A - Joint involvement (0–5 points) | 1 large joint | 0 |
| 2–10 large joints | 1 | |
| 1–3 small joints (large joints not counted) | 2 | |
| 4–10 small joints (large joints not counted) | 3 | |
| >10 joints including at least one small joint | 5 | |
| B - Serology (at least one test needed for classification; 0–3 points) | Negative RF and negative ACPA | 0 |
| Low positive RF or low positive ACPA | 2 | |
| High positive RF or high positive ACPA | 3 | |
| C - Acute-phase reactants (at least one test needed for classification; 0–1 point | Normal CRP and normal ESR | 0 |
| Abnormal CRP or abnormal ESR | 1 | |
| D - Duration of symptoms | 0 | |
| ≥6 weeks | 1 |
Diagnostic role of autoantibodies in RA:
RA is an autoimmune disease associated with the presence of autoantibodies in a majority of patients. These autoantibodies include rheumatoid factor (RF) and anti-protein/citrullinated peptide antibodies (ACPA) for the most studied. The presence of these autoantibodies as well as their levels in the serum play an important role in these diagnostic criteria (Table 3).
Rheumatoid factor
RF is an antibody, most often of the IgM class, directed against the Fc fragment of immunoglobulins. RF are not specific to RA: they can be found in other diseases such as infectious diseases, pneumoconiosis (silicosis, asbestosis), chronic hepatitis (viral, autoimmune), granulomatosis (sarcoidosis) or haematological malignancy. Another example of RF can be found at https://www.immunopaedia.org.za/clinical-cases/infectious-diseases/i-have-spots-and-my-skin-burns/
Anti-protein/citrullinated peptide antibodies
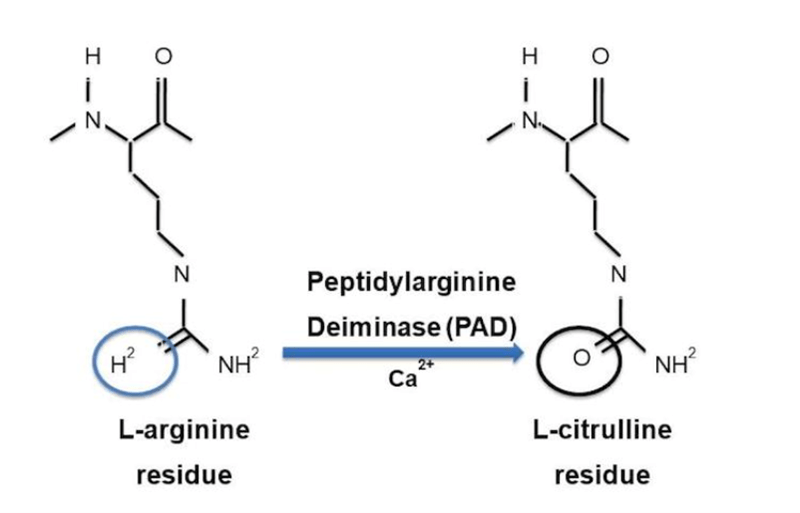
Citrullination is a post-translational modification of the amino acid arginine. It is an enzymatic process catalyzed by peptidyl arginine deiminase (PAD). The transformation of arginine to citrulline generates an intra-articular neo-epitope (Figure 3) which can be recognized by autoantibodies. Citrullination can occur on different proteins (fibrinogen, enolase, histone 2B, vimentin, and filaggrin for example) and the corresponding modified peptides can be presented by antigen-presenting cells, leading to activation of autoreactive T cells. Activated citrullinated peptide-reactive T cells stimulate synoviocytes and macrophages in the joints via proinflammatory cytokines (TNF-α, IL-1 and IL-6). Under the effect of another cytokine, RANKL, osteoclasts are activated and provoke osteoarticular destruction.
Smoking is a major risk factor for RA. It contributes to the citrullination of peptides in the lung by increasing oxidative stress and the generation of citrullinated epitopes. The microbiota (buccal Porphyromonas gingivalis; gut Prevotella copri) with a contribution of exogenous PAD is also responsible for citrullination of peptides in the mucous membranes.
RA is a polygenic disease. The major susceptibility gene is the highly polymorphic HLA-DRB1 gene, with the existence of multiple risk alleles that share a highly conserved 5-aminoacid sequence known as the ‘shared epitope’. The shared epitope is associated with a higher risk of developing ACPA positive RA. It is more prevalent in Caucasian than African populations, explaining in part the lower frequency of RA in the latter than in the former.
The presence of ACPA and RF can be detected several years (10 years on average) before the onset of clinical manifestations of RA.
Experimentally, the disease can be induced in mice (primed at low level with collagen plus adjuvant) with either citrullinated autoantigens or passive transfer of autoantibodies from patients. This indicates the pathogenic character of the citrullinated peptide-specific immune response in RA.
Detection of ACPA and RF
Historical tests for the detection of RF were based on its capacity to agglutinate sheep red blood cells sensitized with rabbit anti-sheep antibodies (Waaler-Rose reaction) or polystyrene particles coated with human IgG (latex test). Methods with higher sensitivity and specificity (see below) are now used for detecting rheumatoid factors. This includes laser nephelometry using the Fc fragment of a human IgG as substrate, and ELISA (Enzyme-Linked Immunosorbent Assay) using the Fc fragment of a rabbit IgG as substrate.
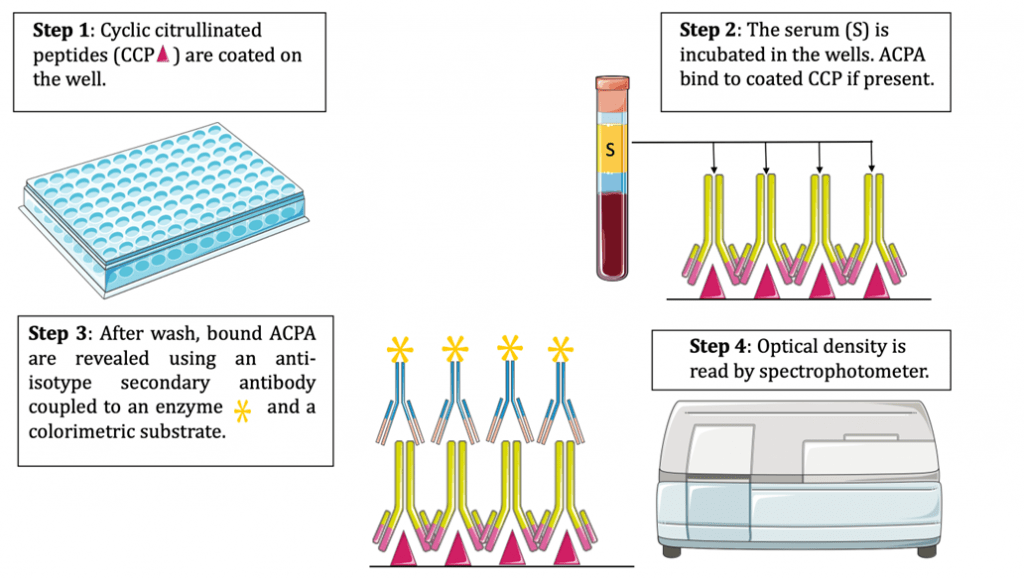
ACPA are currently detected by ELISA, using cyclic citrullinated peptides (CCP) as substrate (Figure 4).
Diagnostic value of autoantibody immunoassays.
Sensitivity, Specificity, Predictive values
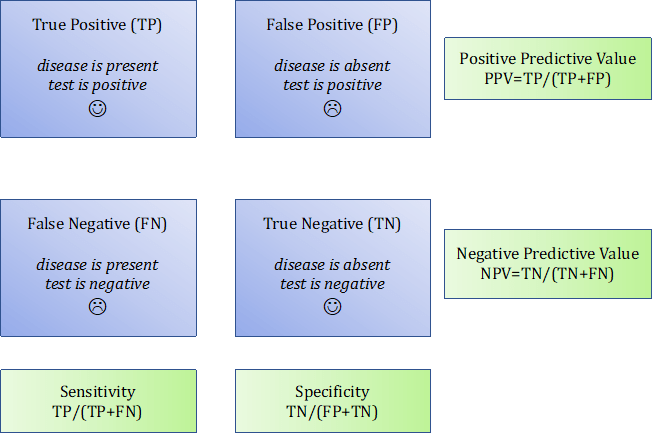
The diagnostic value of a biological test is determined by four parameters (Figure 5):
- Sensitivity: frequency of positive tests in patients (here, suffering from RA).
- Specificity: frequency of negative tests in non-diseased subjects.
- Positive predictive value (PPV): the probability that disease is present in the event of a positive test.
- Negative predictive value (NPV): the probability that disease is absent when the test is negative.
A sensitive test is a test positive in most patients (the rate of false negative results is very low). A test is specific when it concludes appropriately that the disease is present when it is positive (the rate of false positive results is very low).
Developing a diagnostic immunoassay requires performing a clinical study in which the serum of patients (disease is present) and controls (disease is absent) are submitted to the test. Then, the results of the test (positive or negative) determine the clinical status (disease or no disease). In a perfect immunoassay, the test would score positive in every patient and negative in every control. This is rarely 100% the case in practice. Importantly, it is necessary to determine the threshold value of the signal (optical density for ELISA) above which the test is considered as positive. This threshold value has a direct impact on the sensitivity and specificity of the test (Figure 6). Indeed, lowering the threshold augments sensitivity (less risk of false negative results) but reduces specificity (more risk of false positive results). In contrast, augmenting the threshold augments specificity (less risk of false positive results) but lowers sensitivity (more risk of false negative results). In order to determine the best threshold for an immunoassay, computing a ROC curve is essential.
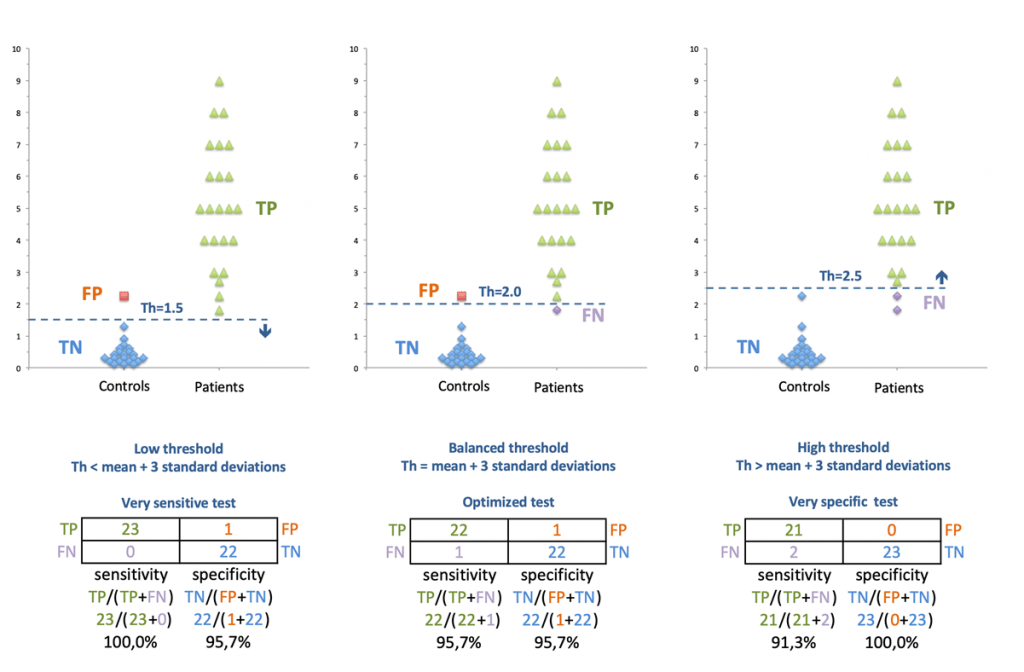
Figure 6: Impact of the threshold choice on sensitivity and specificity of a diagnostic test. TP, true positive; TN, true negative; FP, false positive; FN, false negative.
Receiver Operator Characteristic (ROC) curve
The ROC curve is a tool to analyze the impact of the threshold value on the specificity and sensitivity of the test (Figure 7). The ROC curve graphically depicts the relationship between the sensitivity and specificity of a test across all threshold values. Sensitivity is plotted on the y-axis, while [1 – specificity] is depicted on the x-axis (Figure 7). For each value, a contingency table is computed. By calculating the numbers of true positives (TP), false positives (FP), true negatives (TN) and false negatives (FN), we can determine the sensitivity and specificity of the test. The {[1 – specificity], sensitivity} pairs are then plotted on the curve.
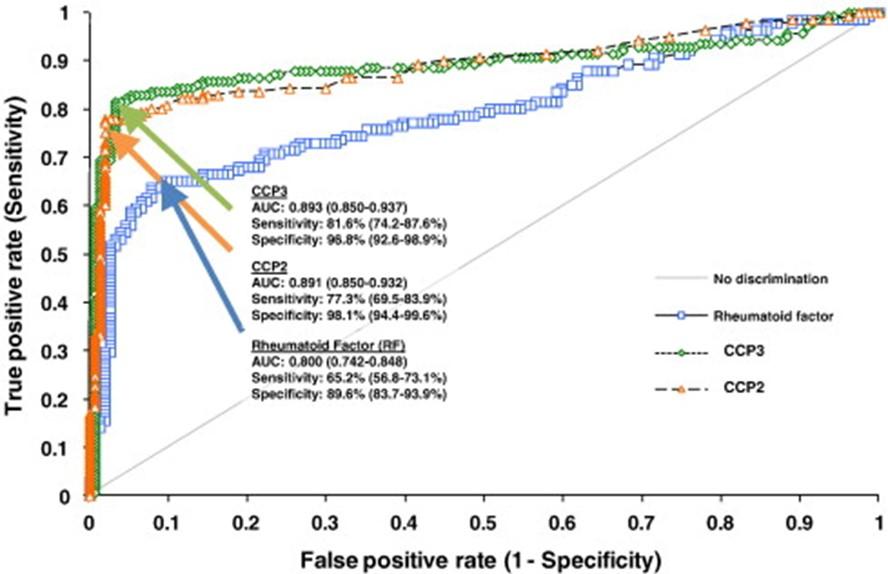
Figure 7: Analysis of the performance of two tests for the detection of ACPA (named here CCP2 and CCP3) as compared to that of a Rheumatoid Factor (RF) test. Sensitivity and [1-Specificity] are plotted on the Receptor Operator Characteristic (ROC) Curve. Both ACPA detection tests showed similar performances (similar areas under the curve, AUC), superior to that of the RF detection test (Swart, Clin Chim Acta 2012; 24:414:266-72)
Sensitivity and specificity of ACPA and RF
Taken separately, ACPA has a sensitivity of 60 to 75% and a specificity of 90 to 95% for diagnosing RA, while RF tests have a sensitivity of 60 to 80% and a lower specificity (around 65 to 85%). The simultaneous presence of ACPA and RF ensures the diagnosis of RA with certainty (specificity of 98%). Besides their diagnostic significance, these two autoantibodies hold prognostic value since they correlate with the severity of joint damage. More than the positivity of ACPA, their level is important. They could be low, moderate, or high. A rate is considered high if it exceeds three times the threshold. Only a high level of ACPA is specific to RA and associated with the positivity of RF. Indeed, there are other states with ACPA positivity: sarcoidosis, psoriatic arthritis, and some lupus. They are associated with erosive polyarthritis. At the onset of initial symptoms of the disease, ACPA and RF are absent in half of the patients, which poses a diagnostic challenge. Furthermore, a fraction of RA patients remain seronegative for both autoantibodies throughout the course of the disease, which may be related to insufficient sensitivity of the ACPA test used for diagnosis. RA with other post-translational modifications like carbamylation or acetylation are not considered in daily practice. This is why the diagnosis of RA does not solely rely on these 2 parameters (Table 2). Indeed, for a patient presenting for the first time with polyarthritis, one of the challenges for clinicians is to rapidly ascertain the diagnosis of RA in order to install an appropriate treatment, which is important because delayed therapy is associated to a poorer response.
!!! ADA stands for “anti-drug antibodies” as mentioned, but some may also use this abbreviation to refer to Adalimumab. Do not get confused!
Treatment
First-line therapy in RA generally relies on the use of non-specific immunosuppressants such as methotrexate, a conventional synthetic Disease-modifying antirheumatic drugs (csDMARD). Understanding the inflammatory mechanisms involved in RA has led to the development of more effective drugs that target key pathophysiological disease pathways. Importantly, TNF-α is the dominant pathogenic cytokine in RA. It can be blocked in vivo using recombinant TNF receptors (like etanercept) or antibodies. Anti-TNF-a therapy is the first-line biologic therapy used here. This choice is motivated by the safety and the extensive use of these molecules for the past 25 years (more than the other drugs used in RA). Monoclonal antibodies targeting TNF-a are categorized as chimeric, humanized or fully human (Figure 8). Chimeric antibodies are composed of murine variable domains and a human constant domain (like infliximab). Because of the presence of non-human domains, they may provoke immunization with the appearance of antidrug antibodies (ADA). Therefore, humanized antibodies (like adalimumab) have been developed that only contain murine sequences in their antigen-binding sites. Consequently, they are less immunogenic but can still lead to an immunization of the patient against the monoclonal antibody. In the case of therapeutic escape, the presence of ADA should be suspected when the drug concentration is found low to negative.
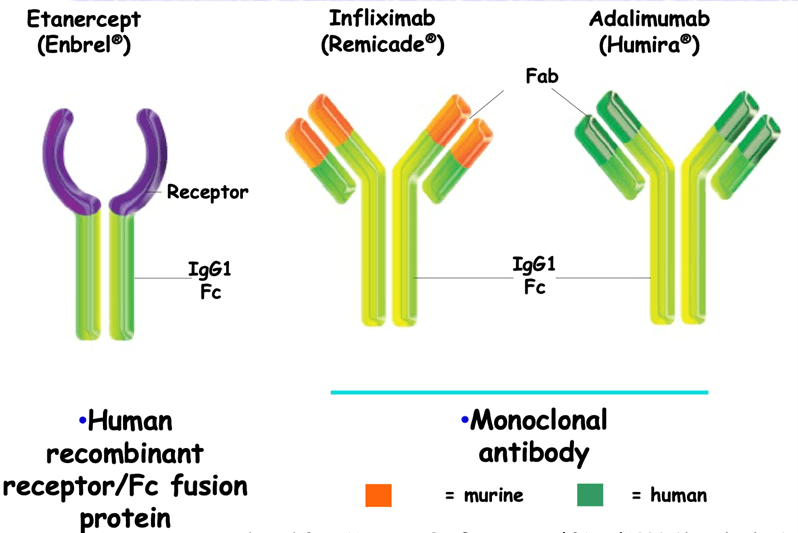
Figure 8: Different types of anti-TNF-: chimeric, humanized, fully human antibodies (Adapted from Hanauer SB, Gastroenterol Disord 2004;4 (Suppl. 3):S18-24)
In the therapeutic strategy of RA, other pathophysiological pathways may be targeted depending on the clinical context:
- targeting IL-6 or its receptor with monoclonal antibodies
- blocking JAK-STAT signalling at the intracellular level using pharmacological inhibitors
- blocking T cell co-stimulation with abatacept, a CTLA4-Ig fusion molecule which competes with the co-stimulatory molecule CD28.
- depleting B cells with an anti-CD20 antibody such as rituximab
Because of their effect on immunity, it is necessary to check, before installing the treatment, for the absence of main bacterial and viral infections such as tuberculosis, B and C hepatitis, and HIV.
Final Outcome
In the presence of clinical aggravation caused by the occurrence of ADA that blocks adalimumab, the rheumatologist decided to introduce weekly subcutaneous 125 mg abatacept. This choice is justified by the recent appearance of RA and the double positivity of ACPA and RF. They are good markers for predicting a positive clinical response to abatacept. Under this therapy, the patient has now been in remission for one year with a DAS28-CRP = 1.5 at the last visit.
References
Kim K, Bang S-Y, Lee H-S, Bae S-C. Update on the genetic architecture of rheumatoid arthritis. Nat Rev Rheumatol 2017;13:13–24.
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023–38.
Minichiello E, Semerano L, Boissier M-C. Time trends in the incidence, prevalence, and severity of rheumatoid arthritis: A systematic literature review. Joint Bone Spine 2016;83:625–30.
Valesini G, Gerardi MC, Iannuccelli C, Pacucci VA, Pendolino M, Shoenfeld Y. Citrullination and autoimmunity. Autoimmunity Reviews 2015;14:490–7.
Evaluation – Questions & answers
Why was RA the final diagnosis of Eva’s disease?
In terms of frequency, RA is the most common inflammatory rheumatic disease, with a prevalence ranging from 0.3 to 1%. The patient’s age, sex, presence of polyarthritis in small joints with a symmetrical distribution, and bone erosions suggest RA. The diagnosis of RA is ascertained by the double positivity of RF and ACPA (specificity close to 98% when both are present) and an ACR/EULAR score ³6 (A5+B3+C1+D1 = 10).
What are the arguments in favour of the autoimmune nature of this disease
Typically, the autoimmune nature of a disease is supported by the Rose and Witebsky criteria:
- Presence of autoantibodies or autoreactive T cells (ACPA in RA),
- The autoantigen is identified (multiple citrullinated antigens in RA: vimentin, α-enolase, histone 2B, fibrinogen),
- Efficacy of immunosuppression (MTX, anti TNF-α),
- Immunization with autoantigen induces disease in animals,
- Passive transfer of autoantibodies from patient to animal induces disease.
Is the presence of rheumatoid factor (RF) in Eva's serum specific to RA?
Rheumatoid factors are not specific to RA. They can be found in the serum of patients suffering from other diseases: infectious diseases, lymphoid diseases, pneumoconiosis (silicosis, asbestosis), chronic hepatitis (viral, autoimmune) or granulomatosis (sarcoidosis).
Why has the patient relapsed after several years of remission on adalimumab treatment?
Adalimumab is not a completely human protein since it retains some murine parts in the antigen binding sites. Therefore, it can be immunogenic in some patients. Eva has developed immunization against adalimumab, as attested by the presence of anti-drug antibodies (ADA) that neutralized this TNF-α inhibitor. This eventually led to a therapeutic escape. That’s why we are switching therapeutic classes. Abatacept appears a good choice since its efficacy has been demonstrated in the recent RA with a double positivity of RF and ACPA.
What should the rheumatologist check before starting anti-TNF-α biotherapy in a patient RA, and why?
TNF-α is a key cytokine for bacterial and viral immunity. The introduction of an anti-TNF-α may lead to a reactivation of a pre-existing latent infection such as tuberculosis, B or C hepatitis or HIV. Therefore, it is essential to check for the absence of these conditions before starting treatment.
What are the x- and y-axis in a ROC curve?
The ROC curve depicts the relationship between the sensitivity and specificity of a test across all threshold values. The y-axis represents sensitivity, while the x-axis corresponds to (1 – specificity).
When developing an immunoassay, what impact has an increase of the threshold value on the specificity and sensitivity of the test?
Increasing the threshold augments specificity (less false positive results) but lowers sensitivity (more false negative results).