- Patient Presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final Outcome
- References
- Evaluation - Questions & answers
Patient Presentation
On 18th July 2006, 6 weeks after introduction of ARVs, an 11 month old baby presented with a one-week history of a right axillary swelling, 2.5cm in diameter noted to be indurated, hard, tender and without fluctuation. By 24th July 2006, the right axillary mass had rapidly enlarged to a size of approximately 4x3cm.
Acknowledgement
This case study was kindly provided by Dr Fatima Laher MBBCh, Dip HIV Man (SA) and Dr Gail Ashford MBBCh, DMH, Dip HIV Man (SA) from the Perinatal HIV research Unit, Chris Hani Baragwanath Hospital, Soweto. Additional input was also provided by Dr Lucy Connell, Harriet Shezi Clinic, Chris Hani Baragwaneth Hospital, Soweto.
History
On 4th June 2005, an HIV-exposed infant was born and received single-dose nevirapine syrup, as well as the standard immunisations including the BCG vaccine. The child was not brought back for an HIV test.
On 3th March 2006, the infant tested HIV positive on two rapid ELISA blood tests, with a CD4 percentage of 9.28 (absolute count 378x10e6/L). Her caregivers defaulted on a referral and telephonic reminders to initiate the child on antiretroviral therapy.
On 10th May 2006, the infant, now 11 months old, presented with a two-week history of diarrhoea and a 4 month history of lethargy and failure to thrive. All immunisations were up to date and no TB contacts were identified.
Differential Diagnosis
- BCG adenitis
- Lymph node tuberculosis
- Pyogenic abscess
- Lymphoma
- Kaposi’s sarcoma
- Immune Reconstitution Inflammatory Syndrome (IRIS)
Examination
Examination on 10 May 2006 revealed the following HIV stigmata:
- oral candidiasis,
- hepatosplenomegaly,
- generalised shotty lymphadenopathy,
- failure to thrive (weight 6.9kg, equivalent to 70% expected weight for age; height 64cm, equivalent to 86% height for age).
Welcome Classification, reference Standard 50th percentile to expected weight:
| 60-80% of standard weight | Less then 60% of standard weight | |
|---|---|---|
| No oedema | Underweight, nutritional dwarfing, growth retardation | Marasmus |
| Oedema | Kwashiorkor | Marasmic kwashiorkor |
In addition to the above there was:
- pallor,
- dehydration,
- pulse rate of 98 b/m,
- pyrexia of 39°C,
- napkin rash,
- loose light-brown stool,
- nitrites on the urine dipstix were observed,
- there was also social, motor and speech developmental regression,
- tympanic membrane red and swollen with visible fluid.
Investigations
| FBC | |
|---|---|
| White cell count | 9.53x10 9 /l |
| Haemoglobin | 5.7 g/dl |
| MCV | 74.4 fl |
| MCH | 21.0 pg |
| Platelets | 397x109/l |
| Differential | Neutrophils=84.6% |
| CD4 | %=0, absolute count=2 cells/ml |
| Viral load | 61 678 RNA copies/ml |
| ALT | 19 u/l |
| Blood culture and Blood TB culture | Negative |
| Chest Radiograph | Normal |
| Tuberculin Skin Test | Negative |
| Gastric washing | AFB negative x 3 |
| Cerebrospinal fluid | Normal cells and chemistry, low adenosine deaminase |
| Bone marrow trephine and biopsy | Features in keeping with HIV |
| Ziehl-Nielsen Stain | Negative |
| TB Culture | Negative |
Histopathology Results:
Fine needle aspiration of Right Axillary Mass:
- Numerous lymphocytes, mature and immature, were seen, with several groups of epitheliod hystiocytes and scattered Langerhans giant cells.
- A single Reed-Sternberg-like cell was noted.
- There were no features of lymphoma or any other malignant infiltrate.
- Scattered acid-fast bacilli were shown on Ziehl-Neelsen stain.
Histology of partial excision biopsy:
- Multiple lymph nodes with granulomatous non-caseating inflammation
- Langerhans type giant cells
- Epithelioid histiocytes
- No features of Hodgkin’s Lymphoma, Kaposis’s or any other malignant infiltrate
Discussion
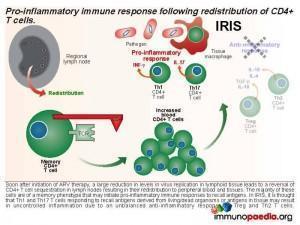
Immune reconstitution inflammatory syndrome (IRIS) is associated with a rapid decline in viral load and release of memory CD4 cells (and hence increase in CD4 peripheral blood count) from sequestered sites. In this case study the child’s prior negative TB investigations and rapid development of an axillary abscess are indicative of an IRIS event.
Although the exact mechanism involved in the development of immune reconstitution inflammatory syndrome (IRIS) is not clearly understood, the explanation presented in this discussion is based on current theories.
As a background to understanding inflammatory responses, it is first important to appreciate that there are four defined CD4+ T cell subsets that provide a homeostatic balance between pro and anti inflammatory responses that occur in a healthy functioning immune system. The four CD4+ T cell subsets we will discuss are the Th1, Th17, Treg and the Th2 subset.
Initially, naive CD4+ T cells (termed Th0) encounter processed antigen by dendritic cells through the MHC class II and T cell receptor in the T cell zone of secondary lymphoid tissues, such as the lymph nodes. Depending on the type of cytokine released by the dendritic cell upon this interaction, the Th0 CD4 cells differentiate into either Th1, Th2, Th17 or Treg phenotypes.
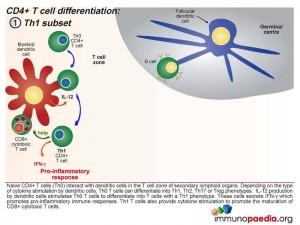
Th1 Subset
Interleukin-12 released by the dendritic cells will cause the Th0 CD4 cells to differentiate into Th1 functional cells. These cells secrete IFN-γ which promotes pro-inflammatory responses and will mobilize the maturation of CD8+ cytotoxic T cells and stimulate NK cells.
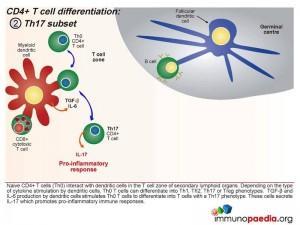
Th17 subset
When a combination of Transforming Growth Factor (TGF β) and IL-6 are released together by the dendritic cell, the Th0 CD4 cells differentiate into Th17 functional cells. These cells secrete IL-17, which also induces pro-inflammatory cytokines and tips the balance towards inflammatory immune responses.
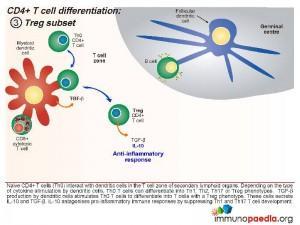
Treg Subset
When the dendritic cells only release TGF- β, the Th0 CD4 cells differentiate into T regulatory (Treg) cells. These cells secrete IL-10 and TGF- β. IL-10 antagonises pro-inflammatory immune responses by suppressing Th1 and Th17 T cell development.
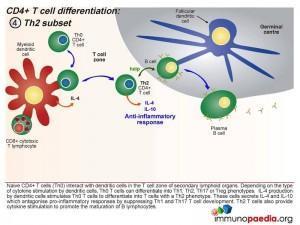
When the dendritic cells release IL-4 after Th0 binding, the cells differentiate into Th2 cells. These cells secrete IL-4 and IL-10 which antagonise pro-inflammatory responses by suppressing Th1 and Th17 T cell development. Th2 T cells also provide cytokine stimulation to promote the maturation of B cells to plasma cells and the production of antibodies.
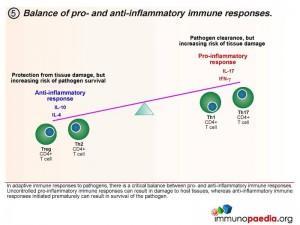
Homeostatic balance of pro- and anti-inflammatory immune responses.
In adaptive immune responses to pathogens, there is a critical balance between pro- and anti-inflammatory immune responses. Uncontrolled pro-inflammatory immune responses can result in damage to host tissues, whereas anti-inflammatory immune responses initiated prematurely can result in survival of the pathogen, which is deleterious for the host. Th1 and Th17 CD4+ T cell subsets result in pro-inflammatory responses and Treg and Th2 CD4+ T cell subsets result in anti-inflammatory responses. It is thought that in a natural healthy immune response, there is a homeostatic balance between these subsets, resulting in the elimination of the pathogen, but also reducing the risk of tissue damage in the process. Inability to clear pathogen, or an overload of antigen, is either a result of this balance or a cause of an imbalance.
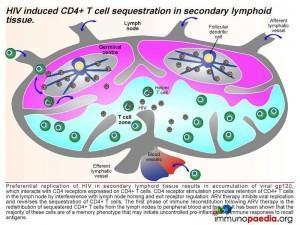
This results in an accumulation of viral gp120, which interacts with CD4 receptors expressed on CD4+ T cells and promotes retention of CD4+ T cells in the lymph node by interference with lymph node homing and exit receptor regulation. When ARV therapy is initiated, the drugs act by inhibiting viral replication and allow the release of sequestrated CD4+ T cells. Thus, the first phase of immune reconstitution following ARV therapy is the redistribution of sequestered CD4+ T cells from the lymph nodes to peripheral blood and tissues. It has been shown that the majority of these cells are Th1 and Th17 memory cells and the second phase is the resulting imbalance towards inflammatory responses due to these cells initiating uncontrolled pro-inflammation.
Download images for this case
Treatment
The child was admitted to the paediatric ward where acute management included a blood transfusion with packed red cells, rehydration, analgesia, a topical antifungal and empiric intravenous treatment for the otitis media and urinary tract infection. Within 5 days the diarrhoea completely resolved and she was no longer noted to have pain on micturition. She was discharged on oral amoxicillin clavulanic acid for completion of treatment of the otitis media and clotrimazole for the fungal skin lesion.
Two weeks after discharge she was started on ARVs stavudine, lamivudine and nevirapine.
Six weeks after starting ARVs she presented with a right axillary swelling which was rapidly enlarging. Mycobacterium bovis was eventually cultured from the fine needle aspiration of the axillary mass. Anti TB treatment was started.
Download images for this case
Final outcome
After two months of treatment nevirapine was switched to efavirenz to prevent drug interactions – specifically hepatotoxicity.
Anaemia resolved and Hb remained within normal limits. The child’s weight increased and she continued to thrive progressing to age-appropriate development.
Download images for this case
References
Sun HY et al. (2009). Immune reconstitution inflammatory syndrome in non-HIV immunocompromised patients. Curr Opin Infect Dis. Aug;22(4):394-402. Review.
Elston JW et al. (2009) Immune reconstitution inflammatory syndrome. Int J STD AIDS. Apr;20(4):221-4. Review.
Mori S et al. (2009). A brief review of potential mechanisms of immune reconstitution inflammatory syndrome in HIV following antiretroviral therapy. Int J STD AIDS. Jul;20(7):447-52. Review.
Download images for this case
Evaluation – Questions & answers
What is the diagnosis in this case?
BCG Adenitis Immune Reconstitution Inflammatory Syndrome (regional disease). Immune Reconstitution Inflammatory Syndrome (IRIS), or Immune Reconstitution Disease (IRD), is defined as paradoxical clinical deterioration (despite decreasing viral load and rising CD4 count) after starting HAART. It results from the interaction between an improving immune system and organisms that colonised the body during earlier stages of HIV infection. Clinical presentations vary depending on the causative organism and the organ system that is colonised. For example, IRIS caused by tuberculosis may present with high fever, lymphadenopathy, worsening of the original tuberculous lesion and/or deteriorating CXR features.
This patient’s presentation, with an acute lymphadenopathy ipsilateral to the BCG inoculation site occurring five weeks after initiation of HAART, is fairly typical of BCG IRIS. In children, IRIS resulting from BCG may occur months to years after vaccination. Most BCG IRIS events occur within the first 3 months of starting HAART and there have been reports of cases occurring as early as a week after initiation of HAART. Less commonly, IRIS can manifest as an occurrence of non-infectious disease, such as Guillain-Barré syndrome. WHO Stage IV disease and a low CD4 (especially CD4 <10% or <50 cells/μl) are risk factors for IRIS development. Patients starting ART in close proximity with diagnosis of an Opportunistic Infection (OI) are at a greater risk of developing IRIS. For example, children who start HAART within two months of starting tuberculosis treatment are more at risk of an IRIS reaction to dead or dying tubercle bacilli
Interpret the abnormal blood findings.
What constitutes the BCG vaccine?
Bacillus of Calmette and Guérin (BCG) is live attenuated Mycobaterium bovis, used as a vaccine against TB. In South Africa, BCG forms part of the Extended Programme of Immunisation and is routinely given at birth.
Special Programme on AIDS and Expanded Programme on Immunization. Joint Statement. Consultation on human immunodeficiency virus (HIV) and routine childhood immunization. Wkly Epidemiol Rec 1987;62:297-99
How is disseminated Mycobacterium bovis treated?
What is the spectrum of BCG adverse events?
The spectrum of BCG adverse reactions includes:
Revised Paediatric BCG Disease Classification (Hesseling et al, 2005)
Local disease:
A local process at the vaccination site, including:
BCG injection site abscess >10mm x 10mm
Severe BCG scar ulceration
Regional disease:
Enlargement, suppuration or fistulation of ipsilateral regional lymph nodes
Distant disease:
Involvement of any site beyond a local or regional ipsilateral process, including:
Pulmonary secretions (gastric washings, tracheal aspirates),
CSF
Urine
Osteitis
Distant skin lesions
Disseminated disease:
BCG confirmed from >1 remote site, as above, and/or from at least 1 blood or bone marrow culture
Other BCG syndromes:
Rare syndromes following vaccination in which bacteria are identified, uveitis and keloid formation
The World Health Organization currently recommends giving BCG to all neonates in areas with a high prevalence of tuberculosis, irrespective of HIV exposure, unless the child has symptomatic HIV disease. HIV-infected infants and other immunodeficient infants are at risk of BCG-related complications. While disseminated disease is rare immunocompetent children, HIV-infected children have significantly increased risk of developing distant or disseminated BCG disease.
What is immune reconstitution inflammatory syndrome (IRIS)?
What factors make a patient more likely to develop an IRIS event?
In what time frame does an IRIS event occur?
Should antiretroviral therapy be stopped or continued if IRIS is suspected/confirmed?
Should the patient be treated for TB?
Should her ARV’s be stopped in the face of rapidly enlarging lymphadenopathy?
Download images for this case