- Patient Presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussions
- Treatment
- Final Outcome
- References
- Evaluation - Questions & answers
- MCQ
Patient Presentation
A three and a half month old boy presents to the emergency room with a 1 day history of apnoea. Earlier in the day he had received his routine immunizations.
Acknowledgement:
This case study was kindly provided by Dr Bhadrish J Mistry, Paediatrician, Department of Paediatrics, Chris Hani Baragwanath Hospital and the University of Witwatersrand.
History
Birth history
- Twin pregnancy
- Normal vaginal delivery at 28 weeks, second twin died in utero.
- Birth weight 1270g.
- Neonatal problems included necrotizing enterocloitis stage 1 (NEC 1), apnoea and anaemia.
Maternal history
- P2G4-1 miscarriage at 12 weeks, 1 full term pregnancy, 1 twin pregnancy
- Rh positive
- Syphilis serology negative.
- HIV negative
Past medical history
Previous admissions:
10 June-15 June
At two and a half months of age the infant was admitted with a urinary tract infection which was identified as ESBL Klebsiella pneumoniae. He was treated and discharged after 5 days.
Differential Diagnosis
- Bronchiolitis
- Asthma
- Croup
- Influenza
- Physiological immunodeficiency
- HIV
- Sepsis
Examination
General
- Weight 2.06 kg, below 3rd centile for weight
- Afebrile
- Blood pressure and heart rate normal
- Mild to moderate respiratory distress
- Mild pallor
- No lymphadenopathy
- No dehydration
- No jaundice
- No stigmata of HIV
Abdomen
No abnormalities detected
Respiratory
Chest clear
Cardiovascular
No abnormalities detected
Neurological
No abnormalities detected
Dermatological
No abnormalities detected
Investigations
| Examination | Value | Normal Limits | |
|---|---|---|---|
| 21 July 13 Aug | |||
| WBC | 16.5 | 9.98 | (4-12 x109/L) |
| HB | 9.9 | 9.5 | (12.1-15.2 g/L) |
| MCV | 83 | 82.7 | (80-96fL) |
| HCT | 0.28999999999999998 | 0.28000000000000003 | |
| Platelets | 617 | 529 | (140-450 x109/L) |
| Neutrophils | 9.57 | ||
| Monocytes | 1.98 | ||
| Lymphocytes | 4.78 | ||
| Eosinophils | 0.17 | ||
| CRP | 105.4 | 67 | |
| Total protein | 47 | 56 | (60-80g/L) |
| Albumin | 29 | 38 | (35-50g/L) |
| Globulin | 16 | 18 | |
| IgG | (4-10) | ||
| IgM | 0.54 | 0.42 | (0.5-2.2) |
| IgA | (0.5-2.2) | ||
| CD3 | 4959 | (1700-3600) | |
| CD3% | 69.2 | ||
| CD4 | 3031 | (1700-2800) | |
| CD4% | 42.5 | ||
| CD8 | 1685 | (800-1200) | |
| CD8% | 24.4 | ||
| CD4:CD8 | 1.8:1 | ||
| CD19 | 1703 | ||
| CD16 | 447 | ||
| CD56 | 447 | ||
| HLA DR | 1942 | ||
| CD40 ligand | positive | ||
| C3 | 0.55000000000000004 | ||
| C4 | 7.0000000000000007E-2 | ||
| HIV Elisa | negative | ||
| Blood Culture | Coagulase negative Staph | ||
| CSF culture | Coagulase negative Staph |
Discussions
Neonatal Immunity
The immune system of neonates is relatively under-developed and relies on maternal antibodies transmitted in utero to modify and control the severity of neonatal diseases. These antibodies are of the class IgG, which can be transported across the placenta . This is an active, selective process that involves intracellular pathways and is specifically mediated by the neonatal Fc receptor FcRn. Fc receptors are expressed by the trophoblastic cell layer which surrounds the developing foetus. Once the receptor has bound IgG it is transported to the foetal circulation. Transfer of antibodies begins at 17 weeks of gestation and increases as gestation advances. Maximal transfer occurs from the third trimester onwards. By 33 weeks of gestation maternal and foetal IgG are at equivalent levels and by 40 weeks of gestation the foetal IgG concentration is higher than maternal IgG. Factors that may reduce transplacental antibody transfer include a) pathogen insult that affect placental integrity, such as HIV or Malaria; b) very high concentrations of maternal IgG which cause antibodies to compete for the receptor and (c) the age of the foetus. Premature infants born before 28 weeks gestation have very low levels of maternal antibodies and prematurity is also responsible for impaired cellular immunity. Having impaired humoral and cellular responses results in a “physiological immunodeficiency” which leaves premature infants vulnerable to both viral and bacterial pathogens as demonstrated in this case study.
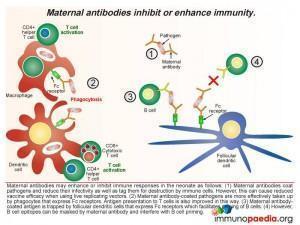
Effects of maternal antibodies
The ways in which maternal antibodies enhance neonatal immunity includes interference with growth of the pathogen and by facilitating organism removal by a process of opsonisation. The antibody-coated pathogens are also more easily taken up by phagocytes that express Fc receptors which improves antigen presentation to T cells. In addition maternal antibody-coated antigen is trapped by follicular dendritic cells that express Fc receptors thus allowing priming of B cells. However there are also times when maternal antibodies can inhibit the immune response in the newborn. Even though it is normal functioning for maternal antibodies to coat pathogens to reduce infectivity and mark them for destruction, this can also bring about the undesired effect of reducing vaccine efficacy when using live replicating vectors because they are rapidly destroyed or growth is impeded (eg. oral polio vaccine or measles virus vaccine). Antibody coating can also mask B cell epitopes thereby interfering with B cell priming.
Along with placental transfer of maternal antibodies, breastmilk is a source of IgA, IgM and IgG antibodies. The Fc receptor is expressed in the lactating breast and serves to transport IgG from maternal blood to breastmilk. There are also B lymphocytes present in breastmilk which can produce antibodies. The Fc receptors expressed on epithelial cells lining the infant gut facilitates transport of maternal IgG into the foetal circulation. IgM and IgA bind antigens present in the gut lumen and small amounts of IgA are also transported into the foetal circulation in the first few days of life.
Immune responses in neonates
Without the passive acquisition of maternal antibodies, the immature nature of neonate humoral and cellular immunity can result in the infant being highly vulnerable to bacterial and viral infections in the perinatal period. Maternal antibodies confer an immunological advantage to the child until the infant immune system has developed to the point of self-sufficiency. The following should be noted about neonatal immunity:
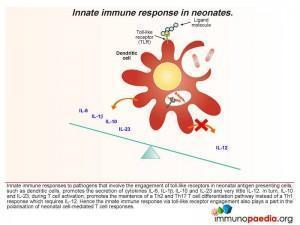
- The innate immune response to pathogens involving engagement of toll-like receptors expressed by neonatal antigen presenting cells, such as dendritic cells, preferentially promote the secretion of cytokines IL-6, IL-1β, IL-10 and IL-23 and very little IL-12. In turn, when T cells become activated, IL-10 and IL-23 favour the maintenance of Th2 (secretion of IL-4 and IL-10) and Th17 (IL-17) helper T cell responses rather than Th1 (secretion of IL-2, IFN-γ) responses.
- The secretion of IL-10 switches off Th1 CD4 cell development and promotes Th2 CD4 cells and counteracts the small amounts of IL-12 produced. Although the neonatal cytokine profile is polarised towards a Th2 response, an overall deficiency of cytokines or co-stimulatory molecules, such as the CD40 ligand, may explain why neonatal CD4 cells have a diminished capacity to provide help to B cells. Engagement of CD40 on B cells is essential for immunoglobulin synthesis, isotype switching and affinity maturation which is impaired in neonates (and hence the importance of maternal IgG).
- The development of anti-viral CD8 cytotoxic T-lymphocytes (CTL) is impaired in infants. For example, fewer infants under 5 months develop CTL responses to respiratory syncytial virus (RSV) compared to those between 6-24 months of age. Hence any viral infection in a child with “physiological immunodeficiency” will be highly vulnerable in the first few weeks of life. It is thought that low expression of surface molecules CD80, CD86 and CD40, which serve as co-stimulatory molecules on activated dendritic cells, fail to provide adequate activation signals for full development of T cells.
- New experimental insights using a mouse model, an additional mechanism contributing to a lack of Th1 responses in the neonatal mouse has been identified and may be present in humans. Neonatal Th1 T cells express a unique receptor for interleukin 4 (IL-4) that induces programmed cell death (apoptosis) when stimulated by IL-4 which is abundantly secreted by activated neonatal Th2T cells.
In the current case, the possibility exists that because the premature infant (born at 28 weeks) possessed low levels of in utero maternal IgG and there was no breastfeeding in the perinatal period, there was a lack of passive transfer of protective maternal IgG. Coupled to impaired neonatal T-dependent B cell responses, as evident from low IgM and IgA antibody titres in the blood (and failure to detect an antibody response to vaccination antigens), this caused the newborn to be highly vulnerable to infections.
Download images for this case
Treatment
- The patient was assessed as a suspected sepsis case and received 5 days of cefotaxime.
- The coagulase negative staph on blood and CSF culture were not treated. Follow up cultures were negative.
- The patient remained infection free until discharge.
- Prior to discharge he received Polygam iv 2g over 24 hours.
- Discharged on 15 August, having been admitted for three and a half weeks.
Download images for this case
Final Outcome
Three days later he was admitted for apnoea secondary to a nosocomial pneumonia. He was treated with tazocin and amikacin for 5 days, responded well and was discharged.
Download images for this case
Kollmann et al. (2009). Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol;183;7150-7160
Lee et al. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J.exp.med;205;10;2269-2280
Englund JA. (2007). The influence of maternal immunization on infant immune responses., J.Comp.Path. 137;S16-S19
Download images for this case
Evaluation – Questions & answers
What is the diagnosis
What is the cause of this condition in this specific case?
How is the developing foetus protected from disease?
What is the mechanism of action of maternally derived antibodies?
What is the mechanism of action of maternally derived antibodies?
What is the purpose of the administration of Polygam in the treatment of the child?
Which type of CD4 helper cell (Th1, Th17 or Th2) is predominant in the developing neonate?
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
Download images for this case