- Patient Presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final Outcome
- References
- Evaluation - Questions & Answers
- MCQ
Patient Presentation
A 7 year old girl was brought in by her mother with a three day history of headache, pain in the left ear, fever and vomiting. Her mother was concerned because the last time she had been sick like this she had to be admitted to hospital.
History
Child was diagnosed as being HIV positive four years ago at age 4 years and has been on HAART since diagnosis.
Currently she has an undetectable viral load and her CD4 count has been increasing(see investigations).
Perinatal History
- Child was born by normal vaginal delivery, full term, with good apgars and no complications were reported. Mother and child were discharged within 24 hours of delivery.
- Child received mixed feeds since birth i.e. breast fed and formula fed.
- Child received all vaccinations, however she was vaccinated on the old immunisation schedule which was prior to the introduction of PCV 7, the polyvalent Pneumococcal vaccine which was introduced into the South African immunisation schedule in 2009.
- Child is thriving with normal weight for age measurements
- Developmentally child has reached all milestones at appropriate intervals
Past Medical History
-
- Child has had six previous hospital admissions for Streptococcus pneumoniae meningitis starting from age 3 years and occurring at approximately six monthly intervals, with the most recent admission having been eight months prior to this presentation.
- On each admission Streptococcus pneumoniae was isolated from cerebrospinal fluid (CSF) sensitive to ceftriaxone. Serotype testing was not available.
- The child was treated with 10 days of IV ceftriaxone and on each occasion CSF was cleared of infection.
- At discharge on all admissions CSF was clear with resolution of meningitis.
- On her third admission at age four she was found to be HIV positive and started on HAART.
- No history of seizures, head trauma or loss of consciousness
Past Surgical History
No history of any surgical procedures.
Family History
-
-
- Father died two years previously of pneumonia
- Mother is HIV positive and has been on HAART for the last 12 months
- One sibling aged 18 months, HIV negative as tested by PCR and is currently well.
-
Social History
The girl lives with mother, three other adults and 4 children (all under 5 years of age) in a two bedroom apartment with electricity and running water.
Differential Diagnosis
-
-
- Localised foci of infection
- Immunodeficiency secondary to HIV despite normal CD4 count
- Primary immunodeficiency
- Community acquired Streptococcus pneumoniae infection
- Defective antibody formation such as agammaglobulinemia or hypogammaglobulinemia
- Defective complement, absent complement or decreased amounts of C1, C2, C3, or C4
- Asplenism
-
Examination
Appearance
-
-
- Ill looking child in obvious discomfort
- Awake and alert, but slow to engage in conversation, GCS 15/15
- Normal weight for age
-
Vitals
-
-
- Temperature: 38.5°C
- Blood pressure 100/68
- Heart rate 110
- Respiratory rate 30
-
General
-
-
- Mild pallor
- Cervical lymphadenopathy
- No signs of dehydration
- No jaundice
- No oedema
-
ENT
Ears:
-
-
- Right ear nothing abnormal detected
- Left ear, inflamed tympanic membrane and accompanying hearing loss
-
Nose and throat:
-
-
- Nothing abnormal detected
-
Respiratory
-
-
- Trachea centrally located.
- Chest clear on auscultation
- No abnormalities detected
-
Cardiovascular
-
-
- No raised JVP
- Normally placed apex beat
- S1 and S2 heart sounds present, no murmurs.
- No abnormalities detected
-
Abdomen
-
-
- Not distended
- Soft and non tender
- Bowel sounds present
- No abnormalities detected
-
Neurological
-
-
- Awake and alert, normal level of consciousness
- Higher function intact
- Photophobia
- ++ Neck stiffness
- Positve Kernig and positive Brudenski signs
- Gait normal
- Power 5/5 globally
- Tone normal globally
- Reflexes 2/4 for both upper and lower limbs
-
Dermatological
-
-
- No rashes noted
-
Investigations
| Examination | Value | Normal Limits |
|---|---|---|
| WBC | 19 | (4-12 x109/L) |
| HB | 14.4 | (12.1-15.2 g/L) |
| Platelets | 220 | (140-450 x109/L) |
| CRP | 65 | (0-8mg/l) |
| NA | 137 | (135-147 mmol/L) |
| K | 4.4000000000000004 | (3.3-5.0 mmol/L) |
| Cl | 98 | (99-103 µmol/L) |
| C02 | 22 | (18-29 mmol/L) |
| Urea | 3.7 | (2.5-6.4 mmol/L) |
| Creatinine | 41 | (62-115 mmol/L) |
| Total protein | 84 | (60-80g/L) |
| Albumin | 41 | (35-50g/L) |
| Total Bilirubin | 8 | |
| Direct Bilirubin | 2 | |
| ALP | 232 | |
| Gamma GT | 20 | |
| ALT | 24 | |
| AST | 26 | |
| Corrected Calcium | 2.46 | (2.1-2.6mmol/l) |
| Phosphate | 0.98 | (1.0-1.5 mmol/l) |
| Magnesium | 0.86 | (0.8-1.3) |
| CSF cell count Polys | 150 | (0 cells) |
| lymphocytes | 45 | (0 cells) |
| erythrocytes | 7 | |
| CSF glucose | 2.9 | (3.3 to 4.4 mmol/l) |
| CSF protein | 1.9 | |
| Bacteria | Streptococcus pneumoniae, sensitive to ceftriaxone | |
| IgG | 12.8 | (4-10) |
| IgM | 1.1299999999999999 | (0.5-2.2) |
| IgA | 1.53 | (0.5-2.2) |
| HIV Elisa | positive | |
| Blood Culture | negative | |
| Complement | ||
| C3 | 1.86 | (0.7-1.6) |
| C4 | 0.35 |
Imaging
-
-
- Skull X ray- normal
- CT Brain- normal
- CT sinuses- normal
- CT Mastoid- shows changes in keeping with left sided mastoiditis
-
Discussion
The girl was diagnosed with Streptococcus pneumoniae (pneumococcus) infection. S. pneumoniae is a gram-positive, encapsulated bacterium, and an important and commonly encountered bacterial pathogen in humans. It is often found as a normal commensal in the nasopharynx of healthy adults and children. It does however have the potential to become pathogenic. In developing countries, including South Africa, pneumococcus remains the most common and important disease-causing organism in infants. Although exact numbers are difficult to obtain, it is estimated that pneumococcal infection is responsible for more than one million of the 2.6 million annual deaths due to acute respiratory infection in children younger than 5 years. Case fatality rates associated with invasive disease vary widely but can approach 50% and are greatest in patients who develop meningitis. The bacterium does so by escaping local host defenses and phagocytic mechanisms, and penetrates the CSF either through choroid plexus/subarachnoid space seeding from bacteraemia or through direct extension from sinusitis, otitis media or mastoiditis. Presently, it is the most common bacterial cause of meningitis, accounting for 47% of cases.
Most individuals who are colonised with pneumococcus carry only a single serotype at any given time. The duration of colonisation varies depending on bacterial characteristics such as serotype and host characteristics. Invasive disease is usually related to recent acquisition of a new serotype. However, in most healthy hosts, colonisation is not associated with symptoms or disease but allows for the continued presence of pneumococcus within the population. The bacterial capsule consists of repeating oligosaccharides and based on antigenic differences within these capsular polysaccharides, greater than 91 serotypes of pneumococcus have been identified. From these a heptavalent pneumococcal conjugate vaccine (PCV7 vaccine) has been designed which contains the 7 most common pneumococcal serotypes causing 70% of the invasive infections in children. With the implementation of childhood vaccinations (since 2000 in USA, 2009 in RSA) using this heptavalent conjugate vaccine (prevnar) for pneumococcus, colonisation rates have decreased in children receiving the vaccine. As a result, adults and other children living in the same household become less exposed and the incident cases of pneumoccocus are significantly reduced because of the phenomenon of herd immunity.
In this discussion we will explore the current understanding of how pneumococcus is able to colonise the nasopharynx, the ways in which the bacterium evades the innate immune system and how it is able to cause disease. With the aid of our graphics we will discuss the processes involved in various bacterial dissemination routes, such as to the middle ear and meninges, dissemination to the lungs and then via the bloodstream to the meninges or dissemination via a bacteraemia to the meninges. We will also go into a more detailed discussion of the strategies employed by the bacterium to evade the innate immune system. Pneumococcal vaccinations will not be discussed in any detail here, but will be detailed in a separate case study.
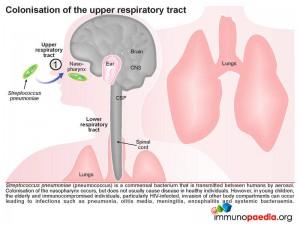
Colonisation of the upper respiratory tract
Pneumococcus is a commensal bacterium that is transmitted between humans via aerosols. In this way the bacteria colonise the nasopharynx, and typically do not cause any disease in healthy individuals. However, amongst children younger then 5 years, the elderly and immunocompromised individuals, especially those who are HIV-infected, asplenic, have complement deficiencies, humoral immunity defects or neutrophil dysfunction, they are all at a greater risk of disease resulting in infections at more distant sites such as pneumonia, otitis media, meningitis, encephalitis and systemic bacteraemia.
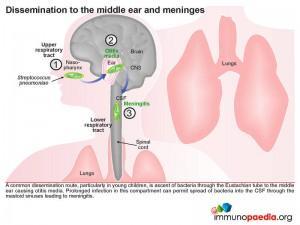 A common dissemination route, particularly in young children, is the ascent of the bacteria via the Eustachian tube to the middle ear causing otitis media. Prolonged infection in this compartment can permit spread of bacteria directly into the CSF through the mastoid sinuses resulting in meningitis. This is the route through which our case study patient was repeatedly infected. In the case of our index patient we know that she cleared infection on every admission. We know that although she is HIV-positive, her immune system is most probably robust due to the viral suppressive effect of HAART (her viral load is undetectable) with a CD4 count in a normal range. We also know from our laboratory investigations that she did not develop a bacteraemia because her blood cultures were always negative therefore her recurrent infections into the meninges was due to direct access to CSF from the middle ear or mastoid sinuses.
A common dissemination route, particularly in young children, is the ascent of the bacteria via the Eustachian tube to the middle ear causing otitis media. Prolonged infection in this compartment can permit spread of bacteria directly into the CSF through the mastoid sinuses resulting in meningitis. This is the route through which our case study patient was repeatedly infected. In the case of our index patient we know that she cleared infection on every admission. We know that although she is HIV-positive, her immune system is most probably robust due to the viral suppressive effect of HAART (her viral load is undetectable) with a CD4 count in a normal range. We also know from our laboratory investigations that she did not develop a bacteraemia because her blood cultures were always negative therefore her recurrent infections into the meninges was due to direct access to CSF from the middle ear or mastoid sinuses.
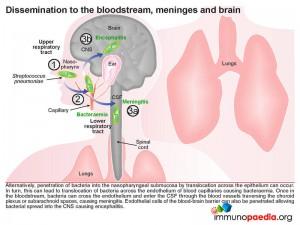
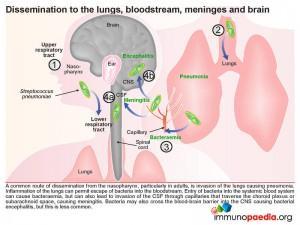 Another common route of dissemination from the nasopharynx, particularly in adults, is seeding of the lower respiratory tract and invasion of the lungs resulting in pneumonia. Inflammation of the lungs can then permit escape of bacteria into the bloodstream. Entry of bacteria into the systemic blood system can result in bacteraemia, but can also lead to invasion of the CSF via the capillary networks that traverse the choroid plexus or subarachnoid space causing meningitis. Alternatively bacteria can penetrate the nasopharyngeal submucosa by translocation across the epithelium and subsequent translocation of capillary endothelial cells entering the blood stream and causing bacteraemia.
Another common route of dissemination from the nasopharynx, particularly in adults, is seeding of the lower respiratory tract and invasion of the lungs resulting in pneumonia. Inflammation of the lungs can then permit escape of bacteria into the bloodstream. Entry of bacteria into the systemic blood system can result in bacteraemia, but can also lead to invasion of the CSF via the capillary networks that traverse the choroid plexus or subarachnoid space causing meningitis. Alternatively bacteria can penetrate the nasopharyngeal submucosa by translocation across the epithelium and subsequent translocation of capillary endothelial cells entering the blood stream and causing bacteraemia.
Once in the bloodstream, bacteria can cross endothelial cell barriers and enter the CSF through the blood vessels traversing the choroid plexus or subarachnoid spaces and cause meningitis. Endothelial cells of the blood-brain barrier can also be penetrated allowing bacterial spread into the CNS, resulting in encephalitis; but this occurs less often.
Now that we understand bacterial dissemination on a gross level we can take a closer look at how the bacteria cross the upper respiratory tract epithelium and translocate the capillary endothelium to gain access to the bloodstream.
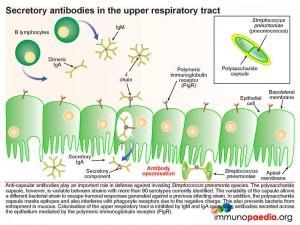
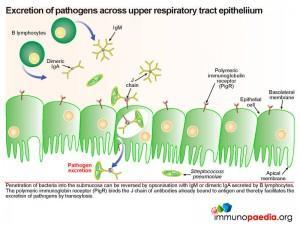
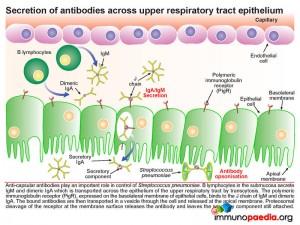 Pneumococcus interferes with the secretory antibody pathway of the upper respiratory track. Anti-capsular antibodies play an important role in control of pneumococcal infections. B lymphocytes in the submucosa secrete IgM and dimeric IgA which is transported across the mucosal epithelium of the upper respiratory tract by transcytosis. The polymeric immunoglobulin receptor (PigR), expressed on the basolateral membrane of epithelial cells, binds to the J chain of IgM and dimericIgA. The bound antibodies are then transported in a vesicle through the cell and released at the apical membrane. Here proteosomal cleavage of the receptor at the membrane surface releases the antibody keeping the secretory component attached.
Pneumococcus interferes with the secretory antibody pathway of the upper respiratory track. Anti-capsular antibodies play an important role in control of pneumococcal infections. B lymphocytes in the submucosa secrete IgM and dimeric IgA which is transported across the mucosal epithelium of the upper respiratory tract by transcytosis. The polymeric immunoglobulin receptor (PigR), expressed on the basolateral membrane of epithelial cells, binds to the J chain of IgM and dimericIgA. The bound antibodies are then transported in a vesicle through the cell and released at the apical membrane. Here proteosomal cleavage of the receptor at the membrane surface releases the antibody keeping the secretory component attached.
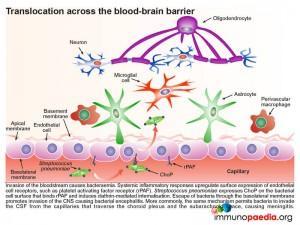
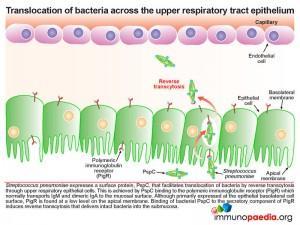 The pneumococcus, which is found in the upper respiratory tract on the apical side, the mucosal epithelium expresses its own surface protein known as PspC. This protein facilitates translocation of bacteria by reverse transcytosis through the upper respiratory epithelial cells. This is achieved by PspC essentially hijacking the polymeric immunoglobulin receptor (PigR) which normally transports IgM and dimeric IgA to the mucosal surface. Although primarily expressed at the epithelial basolateral cell surface, PigR is found at a low level on the apical membrane. Binding of bacterial PspC to the secretory component of PigR induces reverse transcytosis and in this way delivers intact bacteria into the submucosa. PigR is not expressed in the lower respiratory tract and therefore does not play a role in lung disease.
The pneumococcus, which is found in the upper respiratory tract on the apical side, the mucosal epithelium expresses its own surface protein known as PspC. This protein facilitates translocation of bacteria by reverse transcytosis through the upper respiratory epithelial cells. This is achieved by PspC essentially hijacking the polymeric immunoglobulin receptor (PigR) which normally transports IgM and dimeric IgA to the mucosal surface. Although primarily expressed at the epithelial basolateral cell surface, PigR is found at a low level on the apical membrane. Binding of bacterial PspC to the secretory component of PigR induces reverse transcytosis and in this way delivers intact bacteria into the submucosa. PigR is not expressed in the lower respiratory tract and therefore does not play a role in lung disease.
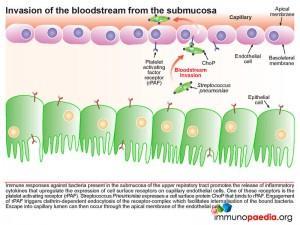 Once in the submucosa the bacteria can further translocate across the capillary endothelium to gain access to the bloodstream. This is achieved via the presence of another cell surface protein expressed by pneumococcus known as ChoP, a phosphorylcholine protein. The bacterium takes advantage of the immune response triggered by its presence in the submucosa of the upper respiratory tract. When inflammatory cytokines are released they upregulate the expression of cell surface receptors on the basolateral surface of the capillary endothelial cells. One of these receptors is the platelet activating factor receptor (PafR).ChoP is able to bind to this receptor and on engagement PafR triggers clathrin-dependent endocytosis of the receptor-complex which facilitates internalisation of the bound bacteria. In this way the bacteria are able to invade the bloodstream from the submucosa via escape at the apical membrane of the endothelial cell into the capillary lumen.
Once in the submucosa the bacteria can further translocate across the capillary endothelium to gain access to the bloodstream. This is achieved via the presence of another cell surface protein expressed by pneumococcus known as ChoP, a phosphorylcholine protein. The bacterium takes advantage of the immune response triggered by its presence in the submucosa of the upper respiratory tract. When inflammatory cytokines are released they upregulate the expression of cell surface receptors on the basolateral surface of the capillary endothelial cells. One of these receptors is the platelet activating factor receptor (PafR).ChoP is able to bind to this receptor and on engagement PafR triggers clathrin-dependent endocytosis of the receptor-complex which facilitates internalisation of the bound bacteria. In this way the bacteria are able to invade the bloodstream from the submucosa via escape at the apical membrane of the endothelial cell into the capillary lumen.
Invasion of the systemic blood system then causes bacteraemia. Systemic inflammatory responses upregulate expression of endothelial cell surface receptors, such as platelet activating factor receptor (PafR), this time on the apical membrane of endothelial cells in the capillary lumen. Since pneumococcus expresses ChoP on the bacterial cell surface which now binds to PafR and induces clathrin-mediated internalization,. escape of bacteria through the basolateral membrane of blood-brain barrier capillaries can promote invasion of the CNS causing bacterial encephalitis. The same mechanism can allow bacteria to invade the CSF from the capillaries that traverse the choroid plexus and the subarachnoid space causing meningitis.
In healthy individuals pneumococcus colonises the nasopharynx without invading the epithelium and becoming pathogenic. However, to maintain survival in the upper respiratory tract and promote invasion of other compartments the bacteria have evolved and developed immune evasion techniques to ensure ongoing survival. Each of these immune evasion strategies are discussed in more detail, below.
Secretory antibodies in the upper respiratory tract
Anti-capsular antibodies play an important role in defense against invading pneumococcal species. The polysaccharide capsule, however, is variable between strains with more than 91 serotypes currently identified. The variability of the capsule allows a different bacterial strain to escape humoral responses generated against a previous infecting strain. In addition, the polysaccharide capsule masks other potential epitopes and interferes with phagocyte receptors due to its negative charge. This negatively charged capsule is also able to prevent entrapment of the bacteria in the naturally occurring mucous lining the nasopharynx. Colonisation of the upper respiratory tract is also inhibited by secretory IgM and IgA opsonising antibodies which are transported across the mucosal epithelium by the polymeric immunoglobulin receptor (PigR).
Excretion of pathogens across upper respiratory tract epithelium
Should the bacteria penetrate into the submucosa this process can be reversed by opsonisation with IgM or dimeric IgA which is secreted by plasma B lymphocytes in the submucosa. Again the polymeric immunoglobin receptor (PigR) plays an important role. Here it binds the J chain of antibodies already bound to bacterial surface antigen and thereby facilitates the excretion of bacteria by transcytosis.
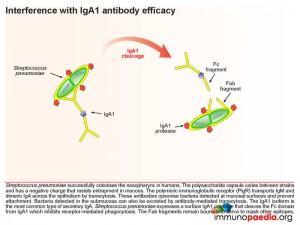
However pneumococcus has also developed a response to interfere with IgA1 antibody (the most common type of secretory IgA) efficiency and expresses a surface IgA1 protease that cleaves the Fc domain from IgA1. In this way it inhibits Fc receptor-mediated phagocytosis. The Fab fragments also remain bound and serve to mask other epitopes.
Complement evasion and inhibition of phagocytosis
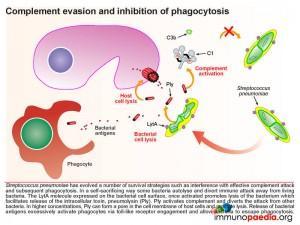
Pneumococcus has also evolved a number of survival strategies which interfere with effective complement attack and subsequent phagocytosis. In a self-sacrificing way, some bacteria autolyse and divert immune attack away from living bacteria. The LytA molecule expressed on the bacterial cell surface, once activated, promotes lysis of the bacterium which facilitates release of the intracellular toxin, pneumolysin (Ply). Pneumococcus isolates produce few toxins however, all serotypes produce Ply. Ply activates complement and diverts the attack from other bacteria. In higher concentrations, Ply can form a pore in the cell membrane of host cells and promote lysis. Release of bacterial antigens excessively activates phagocytes via toll-like receptor engagement and allow bacteria to escape phagocytosis.
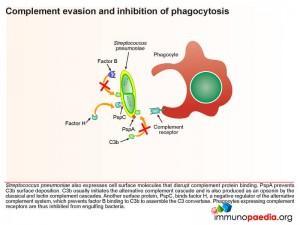 Pneumococcus also expresses cell surface molecules that disrupt complement protein binding. PspA prevents C3b surface deposition. C3b initiates the alternative complement cascade and is also produced as an opsonin by the classical and lectin complement cascades. Another surface protein, PspC, binds factor H, a negative regulator of the alternative complement system, which prevents factor B binding to C3b to assemble the C3 convertase. Phagocytes expressing complement receptors are thereby inhibited from engulfing bacteria
Pneumococcus also expresses cell surface molecules that disrupt complement protein binding. PspA prevents C3b surface deposition. C3b initiates the alternative complement cascade and is also produced as an opsonin by the classical and lectin complement cascades. Another surface protein, PspC, binds factor H, a negative regulator of the alternative complement system, which prevents factor B binding to C3b to assemble the C3 convertase. Phagocytes expressing complement receptors are thereby inhibited from engulfing bacteria
Escape from neutrophil extracellular nets
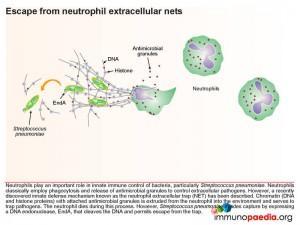
One final immune evasion technique which Streptococcus pneumoniae has adapted is escape from neutrophil extracellular nets. Neutrophils play an important role in innate immune control of bacteria, and classically employ phagocytosis and release of antimicrobial granules to control extracellular pathogens. However, a recently discovered innate defense mechanism known as the neutrophil extracellular trap (NET) explains how chromatin (DNA
and histone proteins) which attach antimicrobial granule proteins is extruded from the neutrophil into the environment and serves to trap pathogens. The neutrophil is sacrificed during this process. However, pneumococcus is able to evade capture by expressing a DNA endonuclease enzyme, EndA, that cleaves the DNA and permits escape from the trap.
All of the above methods of immune evasion show how pneumococcus has evolved to survive as a commensal in the nasopharynx of humans.
Download images for this case
Treatment
A lumbar puncture for CSF examination is urgently warranted in any individual in whom meningitis is clinically suspected. Bacterial meningitis is a neurological emergency that is associated with significant morbidity and mortality. The initiation of empiric antibacterial, antiviral or antifungal therapy is therefore essential for better outcome. This is usually based on the known predisposing factors and/or initial CSF Gram-stain results. Significant delays in instituting antimicrobial treatment in individuals with bacterial meningitis could lead to significant morbidity and mortality. The chosen antibiotic should attain adequate levels in the CSF.
In our clinical case, on all her admissions a lumbar puncture was performed and Streptococcus pneumoniae was isolated. On each occasion the bacteria were found to be sensitive to ceftriaxone a third generation cephalosporin. The patient was treated with intravenous ceftriaxone until the infection was cleared from the CSF. This usually required 10 days of treatment but the most recent admission required 21 days of IV therapy before the infection was cleared.
On this current admission the child has again been treated with IV ceftriaxone. In addition extensive imaging of the sinuses, mastoids, cranium and brain were conducted. The imaging especially CT of the mastoids showed infection in the left mastoid, the usually airfilled honeycomb like appearance of the cavities were obliterated. Based on these findings and the patients history of recurrent meningits the child was scheduled for a left sided mastoidectomy as this was believed to be the source of direct inoculation of Streptococcus pneumoniae into the CSF.
Prophylactic antibiotics were prescribed until the time of surgery to prevent any further recurrent infections.
It was decided that although the child had not received the heptavalent pneumococcal conjugate vaccine (PCV7 vaccine) because it was not part of the vaccination schedule when she was an infant and toddler, there would be no additional benefit in giving it to her at this stage. The reason for this is that her immune system is currently functioning well which means that she has already formed antibodies to Streptococcus pneumoniae. The reason for her recurrence of infection was not due to a poor immune response but rather to a direct inoculation of bacteria into the CSF via otitis media and mastoiditis.
Download images for this case
Final Outcome
Following the mastoidectomy, the patient has had no further recurrence of ear infections or Streptococcus pneumoniae meningitis. She continues on HAART and her viral load has remained undetectable. Her CD4 count continues to rise slowly.
She continues to thrive and has reached all milestones developmentally and socially. However audiometry testing has showed 30% hearing loss in her left ear compared to her right ear.
Download images for this case
References
Kadioglu A et al. (2008) The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. Apr;6(4):288-301. Review.
Mitchell AM, Mitchell TJ. (2010) Streptococcus pneumoniae: virulence factors and variation. Clin Microbiol Infect. May;16(5):411-8. Epub 2010 Feb 2. Review
Kaetzel CS. (2001) Polymeric Ig receptor: defender of the fort or Trojan horse? Curr Biol. Jan 9;11(1):R35-8.
Pickering LK et al. (2009) Committee on Infectious Diseases; American Academy of Pediatrics. Pneumococcal Infections. Red Book 2009 Report of the Committee on Infectious Diseases. 28th. American Academy of Pediatrics;525-335.
Musher DM (2005). Streptococcus pneumoniae. In: Mandell GL, Bennett JE, Dolin R. Principles and Practice of Infectious Diseases. 2. 6th. Philadelphia, Pennsylvania: Elsevier, Churchill Livingstone;197.
Rudan I, Campbell H. ( 2009) The deadly toll of S pneumoniae and H influenzae type b. Lancet. Sep 12;374(9693):854-6.
Download images for this case
Evaluation – Questions & Answers
What is the diagnosis?
What is the cause of the recurrent infections in this patient?
What allows this bacteria which is typically a commensal in the nasopharynx of humans to become pathogenic?
There are various innate immune system factors against which Streptococcus pneumoniae has successfully evolved defenses allowing the bacteria to colonise the nasopharynx in humans. These include:
- the polysaccharide capsule which contains a negative charge and is thus able to resist entrapment in mucous.
- The IgA1 protease expressed by Streptococcus pneumoniae cleaves the Fc domain from secretory IgA1 thereby inhibiting phagocytosis.
- The release of pneumolysin from the autolysed bacteria activates complement and diverts the attack from live bacteria.
- Disruption of complement protein binding by bacterial surface molecules results in prevention of C3b surface deposition, thus preventing C3 convertase formation and inhibiting phagocytosis.
- Neutrophils which typically phagocytose bacteria, also use a neutrophil extracellular trap to capture bacteria. However this is prevented from by pneumococcal production of a DNA endonuclease enzyme called EndA. This enzyme cleaves the DNA found in chromatin structures, which are normally extruded from the neutrophil into the environment to trap pathogens. By cleaving the DNA the bacteria are able to escape from the trap.
Who is susceptible to infections with this bacteria?
What changes occur to first allow this bacteria to invade the submucosa of the upper respiratory tract and then the bloodstream?
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
Download images for this case
Dr Jerusha Naidoo, Southern Africa Regional Vaccines Medical Lead at Pfizer – Pneumococcal Vaccines