- Patient Presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final outcome
- References
- Evaluation - Questions & answers
- MCQs
Patient Presentation
Elsa, 25 years-old, teacher, complains of double vision. She finds it difficult to talk after prolonged speaking. She is pregnant.
Acknowledgements
This case study was provided by Prof. Olivier Boyer (M.D., Ph.D., Head of the Department of Immunology, Rouen University Hospital, France) and Dr. Audrey Aussy (M.D., Assistant Professor) of the Faculty of Medicine of Rouen, Normandy University, France. The authors would like to thank Bertrand Bourre, Lucie Guyant-Maréchal, Rozen Le Panse and François Tron for their critical reading of this case study. We are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript.
Partnership
We have partnered with The International Union of Basic and Clinical Pharmacology (IUPHAR) to bring you in-depth information about drugs and pharmacology with links to the Guide to ImmunoPharmacology.
History
Episodes of double vision began 6 weeks ago. Elsa’s husband reported fluctuating droopy eyelids, in the morning and evening, which started 4 weeks ago. She described asthenia (lack of energy) and generalized weakness when gardening. Symptoms are worst at the end of the day.
Past medical history
- None
- No allergies
Past Surgical History
- Appendix removed 10 years ago
Family History
- Her mother is dyslipidemic (Abnormally elevated cholesterol or lipids in the blood)
- Her sister has type 1 diabetes
Travel history
- She traveled to Italy 2 years ago
Social history
- Teacher
- Pregnancy (26 weeks LMP (last menstrual period))
Medication
- None
Differential Diagnosis
- Brain tumor
- Myasthenia Gravis
- Lambert-Eaton syndrome
- Botulism
- Myositis
- Vertebro-basilar insufficiency
- Multiple sclerosis
- Graves’ disease
- Chronic progressive external ophthalmoplegia (paralysis of the muscles within or surrounding the eye)
Examination
Vitals
- Heart rate: 76/min
- Blood pressure: 124/82 mmHg
- Temperature: 36.8°C
- Oxygen saturation: 98%
General
- She looks tired
- No pallor
- No clubbing, no rash
- No cough
Cardiovascular
- Normal heart sounds
- All pulses present
Respiratory
- No dyspnea (laboured breathing), respiratory rate 17/min
- No chest deformity
- Good bilateral air entry
- No crackling sound on the lungs or wheezing
Abdomen
- Normal
Neurological
- Bilateral ptosis (drooping eyelids)
- Limitations in eye movements of both eyes when looking to the right and to the left
- No paralysis of other cranial nerves
- Normal pupillary reflexes
- Normal reflexes on upper and lower limbs
- Intact sensation, vibration sense and proprioception (The ability to sense stimuli arising within the body regarding position, motion, and equilibrium)
- No sphincter disorder
- Normal cognition
- No meningism
Musculoskeletal system
- Normal axial tone
- Normal power of lower and upper limbs
- Waddling gait after 100 m
- Need to use hands to stand up after 5 squats
Follow-up clinical examinations
Twelve weeks later, Elsa delivers a 3.1 kg newborn, Elvis.
Ten hours after his birth, Elvis presents hypotonicity (less than normal muscle tone) and poor sucking. There is no respiratory failure.
Three weeks after Elvis’ birth, Elsa consults for exacerbation of diplopia (double visioon) and ptosis. She describes axial and limb weakness. She also presents difficulty in swallowing food. Examination reveals hypotonicity of head and strength 3/5 of the four limbs (movement possible against gravity, but not against resistance by the examiner). She is admitted to hospital. Her vital capacity (amount of air she can exhale in one deep breath) is low, at 3.2 liters.
Investigations
Biological investigations did not reveal abnormalities in CBC, electrolytes, kidney function and inflammatory proteins. Immunological analyses evidenced the absence of anti-nuclear aAb and of myositis-specific aAb. In contrast, anti-AchR aAb scored positive while other MG-associated (anti-MusK and anti-LRP4) aAb were negative.
Examination Value Normal Limits
| Examination | Value | Normal limits |
|---|---|---|
| WBC | 7.41 | (4-12 x10 9 /L) |
| HB | 13.8 | (12.1-15.2 g/L) |
| Platelets | 152 | (140-450 x10 9 /L) |
| CRP | 5 | (0-8 mg/l) |
| Na | 137 | (135-147 mmol/L) |
| K | 4.2 | (3.3-5.0 mmol/L) |
| Cl | 101 | (99-103 μmol/L) |
| Urea | 6.2 | (2.5-6.4 mmol/L) |
| Creatinine | 68 | (62-115 mmol/L) |
| CK | 183 | (25-195 IU/L) |
| Total protein | 72 | (60-80 g/L) |
| Albumin | 42 | (35-50 g/L) |
| Corrected calcium | 2.2 | (2.1-2.6 mmol/L) |
| Phosphate | 1.4 | (1.0-1.5 mmol/L) |
| Magnesium | 1.1 | (0.8-1.3 mmol/L) |
| Thyroid stimulating hormone | 11.3 | (9-30 mIU/L) |
| Free T4 | 14.2 | (10-26 pmol/L) |
| Antinuclear antibodies | Negative | |
| Myositis-specific antibodies | All negative | |
| C3 | 1.17 | (0.5-1.53 g/L) |
| C4 | 0.9 | (0.2-1 g/L) |
| Anti-acetylcholine receptor antibodies | 7.50 | (less than 0.5 nmol/L) |
| Anti-MuSK antibodies | Negative | |
| Anti-LRP4 antibodies | Negative |
EKG: normal
Chest X-ray: enlargement of upper mediastinum
Chest computed tomography: presence of mildly, smooth margin, enhanced mass in the anterior mediastinum, without invasion of adjacent organs
EMG: decrementing response to 3 Hz repetitive nerve stimulation
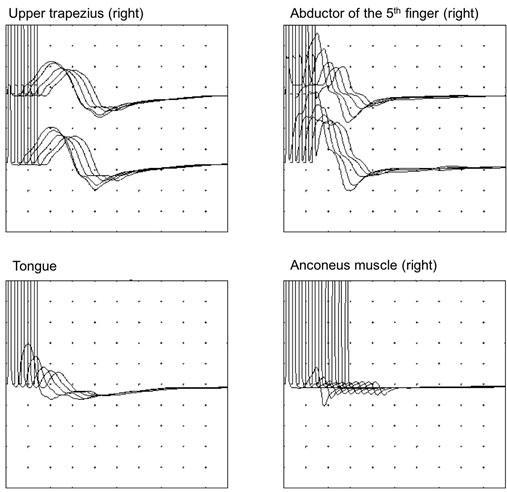
EMG : repetitive nerve stimulation was performed 5-10 times at 3 Hz and muscle action potentials were recorded with surface electrodes taped to skin in regard of muscle of interest. Repetitive nerve stimulation provoked a >10% reduction in amplitude between the first and the fourth muscle action potential. [Image was kindly provided by Lucie Guyant-Marechal]
Discussion
The cause of the presenting condition is Myasthenia Gravis (MG).
MG is a disease characterized by fluctuating weakness of striated muscles that worsens with activity. The weakness is due to impaired neuromuscular transmission resulting from a decrease of receptors for acetylcholine (ACh) at the post-synaptic junction. This reduction is caused by autoantibodies directed against the acetylcholine receptor (anti-AChR).
In healthy individuals, repetitive nerve stimulation results in the release of decreased amounts of ACh at the neuromuscular junctions with each successive stimulus. Yet, enough ACh is released to permit muscle contraction.
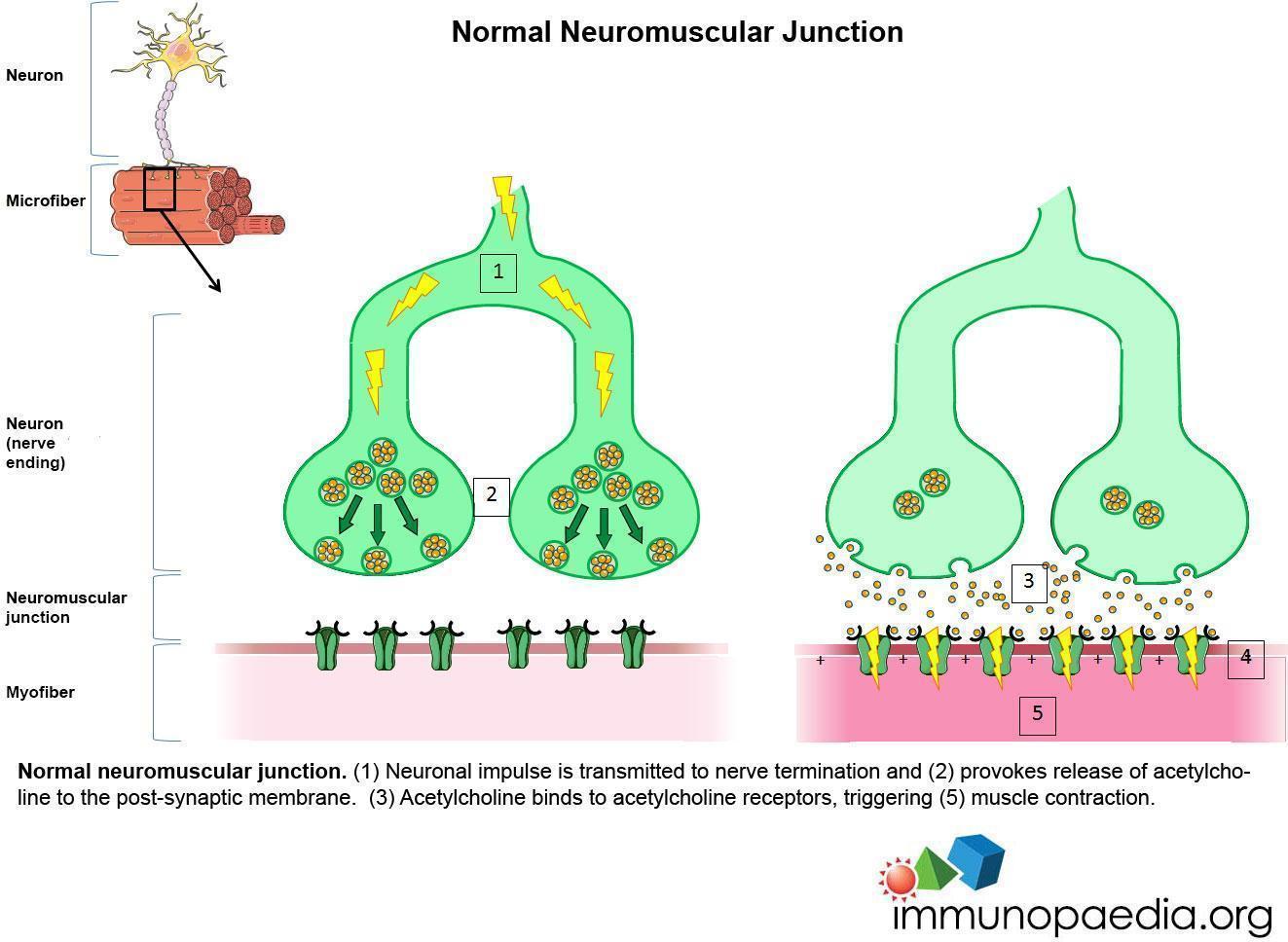
Normal neuromuscular junction. (1) Neuronal impulse is transmitted to nerve termination and (2) provokes release of acetylcholine to the post-synaptic membrane. (3) Acetylcholine binds to acetylcholine receptors, triggering (5) muscle contraction.
MG pathogenesis is mediated by anti-AChR autoantibodies through three mechanisms. The predominant mechanism of injury to the neuromuscular junction is complement-mediated lysis of the postsynaptic membrane. Anti-AChR antibodies can also block AChR, preventing the binding of Ach to its receptor, or provoke internalization of AChR, resulting in a reduction of AChR number at the synaptic junction. The overall consequence is an alteration of neurotransmission that rapidly aggravates under repetitive stimulation, causing the typical trait of MG, i.e. worsening under activity. Eyelid muscles are particularly vulnerable to this process, because of their frequent movements.
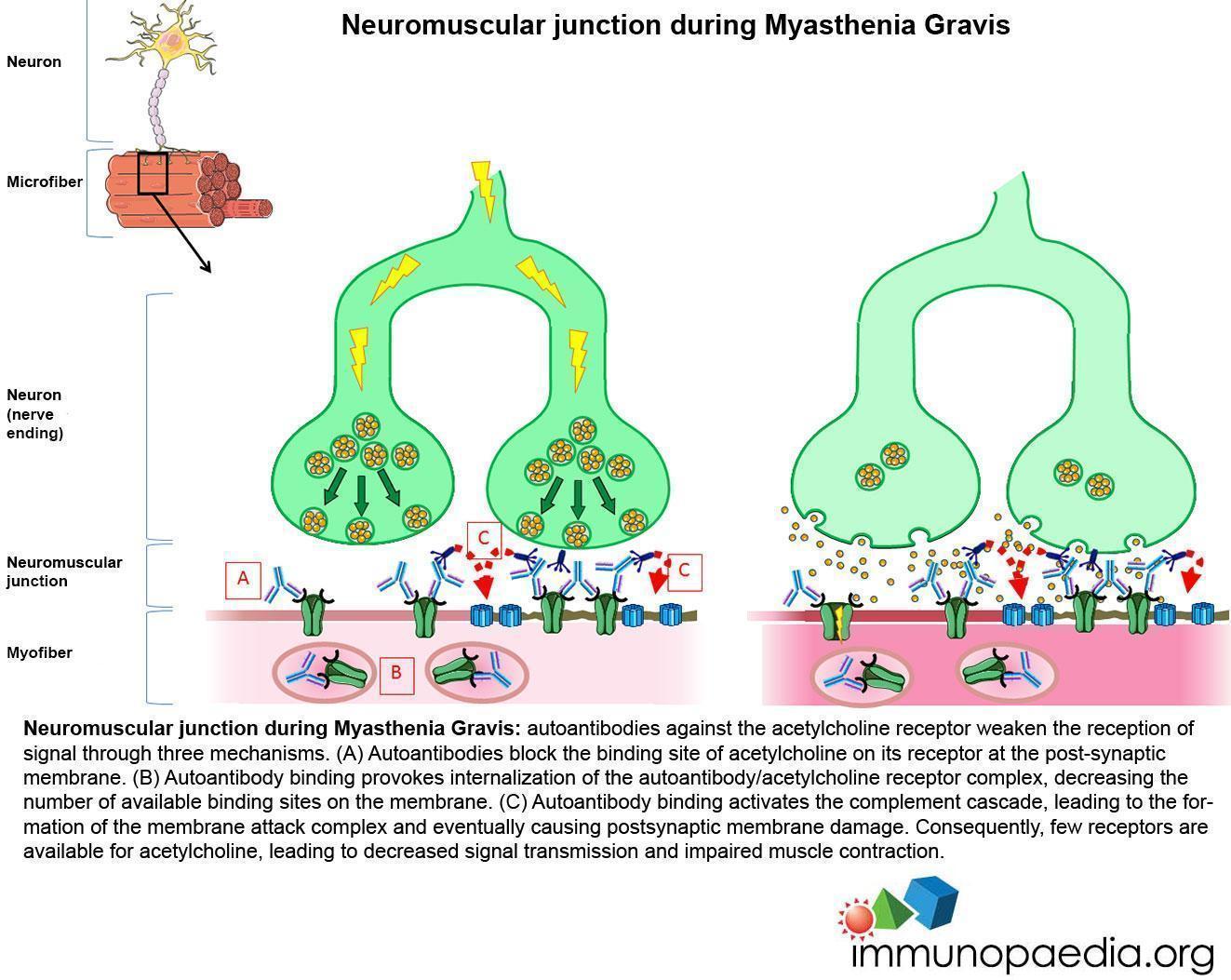
Neuromuscular junction during Myasthenia Gravis: autoantibodies against the acetylcholine receptor weaken the reception of signal through three mechanisms. (A) Autoantibodies block the binding site of acetylcholine on its receptor at the post-synaptic membrane. (B) Autoantibody binding provokes internalization of the autoantibody/acetylcholine receptor complex, decreasing the number of available binding sites on the membrane. (C) Autoantibody binding activates the complement cascade, leading to the formation of the membrane attack complex and eventually causing postsynaptic membrane damage. Consequently, few receptors are available for acetylcholine, leading to decreased signal transmission and impaired muscle contraction.
The incidence ranges from 0.3 to 2.8 per 100,000, corresponding to a prevalence of 700,000 patients worldwide. Disease could start at any age of life, but preferentially affects women under 40. After the 6th decade, there is male predominance. Many studies have assessed the involvement of genetic loci in MG susceptibility, mostly HLA alleles according to ethnicity but also some non–HLA genes such as PTPN22 or CTLA-4.
Elsa developed a moderate but generalized myasthenia at initial point. Pregnancy frequently increases the risk of crisis. After giving birth, she developed a severe crisis of myasthenia. Crises are triggered by different factors: infections, stress, drugs etc. The perinatal period may be a trigger factor. Evolution of MG is capricious and frequently characterized by the alternation of outbreak and remission. Maximal severity of MG occurs in the first three years for 85% of patients. The appearance of respiratory troubles and difficulty swallowing may be fatal and impose hospitalization in intensive care units.
Diagnosis is based on clinical and electrophysiological data. Suggestive clinical signs are diplopia, ptosis, snuffling voice, wrong-way swallowing and weakness, with circadian fluctuations and post-exertional aggravation. Specific signs on electromyogram include a decrementing response > 10%, concerning at least 2 couple muscles/nerves, during a 3 Hz repetitive nerve stimulation. The effectiveness of anticholinesterasics is another element for diagnosis.
The Myasthenia Gravis Foundation of America (MGFA) clinical classification is useful to score the severity of disease and to determine the therapeutic strategy in accordance with international consensus guidance. Goals of treatment are to obtain the minimal manifestation (MM) status, as defined by the MGFA Post-intervention Status, with no more than grade 1 medication side effects.
MGFA Clinical Classification
Class I:
Any ocular muscle weakness; may have weakness of eye closure. All other muscle strength is normal.
Class II:
Mild weakness affecting muscles other than ocular muscles; may also have ocular muscle weakness of any severity.
- A) IIa. Predominantly affecting limb, axial muscles, or both. May also have lesser involvement of oropharyngeal muscles.
- B) IIb. Predominantly affecting oropharyngeal, respiratory muscles, or both. May also have lesser or equal involvement of limb, axial muscles, or both.
Class III:
Moderate weakness affecting muscles other than ocular muscles; may also have ocular muscle weakness of any severity.
- A) IIIa. Predominantly affecting limb, axial muscles, or both. May also have lesser involvement of oropharyngeal muscles.
- B) IIIb. Predominantly affecting oropharyngeal, respiratory muscles, or both. May also have lesser or equal involvement of limb, axial muscles, or both.
Class IV:
Severe weakness affecting muscles other than ocular muscles; may also have ocular muscle weakness of any severity.
- A) IVa. Predominantly affecting limb, axial muscles, or both. May also have lesser involvement of oropharyngeal muscles.
- B) IVb. Predominantly affecting oropharyngeal, respiratory muscles, or both. May also have lesser or equal involvement of limb, axial muscles, or both.
Class V:
Defined as intubation, with or without mechanical ventilation, except when employed during routine postoperative management. The use of a feeding tube without intubation places the patient in class IVb.
MGFA Post-intervention Status
Complete Stable Remission (CSR):
The patient has had no symptoms or signs of MG for at least 1 year and has received no therapy for MG during that time. There is no weakness of any muscle on careful examination by someone skilled in the evaluation of neuromuscular disease. Isolated weakness of eyelid closure is accepted.
Pharmacologic Remission (PR):
The same criteria as for CSR except that the patient continues to take some form of therapy for MG. Patients taking cholinesterase inhibitors are excluded from this category because their use suggests the presence of weakness.
Minimal Manifestations (MM):
The patient has no symptoms of functional limitations from MG but has some weakness on examination of some muscles. This class recognizes that some patients who otherwise meet the definition of CSR or PR do have weakness that is only detectable by careful examination.
- MM-0: The patient has received no MG treatment for at least 1 year.
- MM-1: The patient continues to receive some form of immunosuppression but no cholinesterase inhibitors or other symptomatic therapy.
- MM-2: The patient has received only low-dose cholinesterase inhibitors (<120 mg pyridostigmine/day) for at least 1 year.
- MM-3: The patient has received cholinesterase inhibitors or other symptomatic therapy and some form of immunosuppression during the past year.
Change in Status
Improved (I): A substantial decrease in pretreatment clinical manifestations or a sustained substantial reduction in MG medications as defined in the protocol. In prospective studies, this should be defined as a specific decrease in QMG score.
Unchanged (U): No substantial change in pretreatment clinical manifestations or reduction in MG medications as defined in the protocol. In prospective studies, this should be defined in terms of a maximum change in QMG score.
Worse (W): A substantial increase in pretreatment clinical manifestations or a substantial increase in MG medications as defined in the protocol. In prospective studies, this should be defined as a specific increase in QMG score.
Exacerbation (E): Patients who have fulfilled criteria of CSR, PR, or MM but subsequently developed clinical findings greater than permitted by these criteria.
Died of MG (D of MG): Patients who died of MG, of complications of MG therapy, or within 30 days after thymectomy (removal of the thymus gland).
Elvis developed neonatal myasthenia as he was unable to produce his own immunoglobulins at birth. This form is due to the passive transfer of pathogenic IgG from his mother through the placenta during pregnancy. Only IgG can cross the placenta. Symptoms of MG disappear with IgG after 2 to 3 weeks in the newborn, in accordance with a lifetime of IgG. There is no correlation between onset of neonatal myasthenia and severity of mother’s disease.
Elsa’s chest X-ray and tomography found a mass in the anterior mediastinum. MG is very often associated with thymus structural and functional abnormalities in the majority of anti-AChR+ patients, with 15-20% of thymic tumors (thymoma) and 50-60% of thymic hyperplasia (abnormal increase in organ volume) of lymphoproliferative origin. Thymoma is often observed after the age of 45-50 years. Thymectomy is necessary to control thymoma but does not particularly improve MG symptoms. MG associated with thymoma abnormalities is commonly generalized and more severe, requiring more frequently immunosuppressive treatments. Thymic hyperplasia is an early-onset form of the disease usually observed before the age of 45 years and affecting mainly women. Thymectomy is recommended to reduce the severity of MG and leads to remission and decreasing level of anti-AChR in a significant proportion of patients, suggesting a causal relationship between the pathological thymus and the onset of MG.
The hyperplastic MG thymus is characterized by the presence of multiple germinal centers (GC), which are the site of intense B-cell proliferation, differentiation and selection. These GC contain the actors of the anti-AChR immune response: B cells giving rise to plasma cells producing the anti-AChR antibodies, autoreactive T cells, antigen-presenting cells and the autoantigen (expressed by thymic epithelia cells and myoid cells). Recent data suggests that the thymus becomes a tertiary lymphoid organ (TLO), inasmuch as non-lymphoid tissues are exposed to chronic inflammation in other autoimmune diseases. A TLO is characterized by the presence of GC, a particular vascular network, inflammatory cytokines and other leukocytes. In MG, the thymus harbors plasma cells that produce autoantibodies targeting different epitopes of AChR, and autoreactive T cells. In thymoma, the presence of autoreactive T cells has been associated with the decreased expression of AIRE (AutoImmune REgulator gene) with lower numbers of regulatory T (Treg) cells, resulting in impaired negative selection and defective regulation of AChR-specific T cells. In the early-onset form of the disease, the chronic inflammation present in the thymus of MG patients could result in upregulation of both chemokines and cytokines involved in cell recruitment, vascular networking and AChR presentation to lymphoid cells. The T cell phenotype is affected with abnormal expression of IL-17-related genes in Treg cells and increased expression of interferon-γ, IL-21 and TNF-α in both Treg and effector T cells.
Alternative autoantibodies that may be found in MG patients are:
- Anti-MuSK (muscle specific kinase), a tyrosine-phosphokinase implicated in the clusterization of AChR at the neuro-muscular junction.
- Anti-LRP4 (low-density lipoprotein receptor-related protein 4), the agrin-receptor, involved in MuSK activation.
Examples of other autoimmune diseases in which autoantibodies exert a pathogenic role are numerous. For instance:
- binding of activating autoantibodies to the TSH receptor results in hyperthyroidism in Graves’ disease,
- anti-DNA autoantibodies form immune complexes that deposit and activate complement in kidneys of patients with systemic lupus erythematosus,
- anti-desmoglein autoantibodies dissociate epidermal cells in pemphigus,
- autoantibodies to red blood cells may activate antibody-dependent cell cytotoxicity (ADCC) in spleen of patients with autoimmune hemolytic anemia.
Download images for this case
Treatment
Patient’s treatment plan
Elsa was initially treated symptomatically with pyridostigmine, an inhibitor of cholinesterase. This drug augments the half-life of Ach at the synaptic junction. Due to side effects (diarrhoea, cholinergic syndrome) that occurred after childbirth, she decreased the dosage. This resulted in symptom exacerbation and hospitalization. Azathioprine and corticosteroids were started and she received intravenous immunoglobulin 0.4 g/kg/24h for 5 days.
Recommended treatments
Objective: to obtain minimal MGFA Post-intervention Status (no symptoms or functional limitations but some weakness on examination) without presenting side effect more than grade 1.
Symptomatic treatment: inhibitor of cholinesterase (for instance, pyridostigmine) is initially prescribed in each patient with suspected or confirmed myasthenia, whatever the severity. The dose should be adjusted as needed, based on symptoms.
Immunosuppressive treatments (IS) are indicated in all patients with MG insufficiently improved with anticholinesterasics (when the objective of MGFA Post-intervention Status is not reached). Corticosteroids constitute first choice of IS. Nonetheless, nonsteroidal IS should be used alone in first line when corticosteroids are contraindicated or refused. Corticosteroids and non-steroidal agents should be associated when i) risk of steroid side effects is high considering medical comorbidities; ii) corticosteroid side effects develop; iii) response is inadequate; and iv) the corticosteroid dose cannot be reduced due to symptom relapse. The first nonsteroidal IS recommended is azathioprine. Mycophenolate mofetil, cyclosporine, methotrexate and tacrolimus constitute the main alternatives.
Patients with refractory MG have to be referred to an expert center or physician. Refractory MG is defined by unchanged MGFA Post intervention Status after corticosteroids and at least two other IS agents. Second line treatments comprise IVIg, plasma exchange, cyclophosphamide or rituximab.
Once the goal of treatment is achieved, corticosteroids are gradually tapered. Nonsteroidal immunosuppressive drugs are generally maintained for months or years. The physician may try to stop them after a long period but there are no guidelines.
Treatment of MG crisis is an emergency. Hospitalization is required to immediately start short-term plasma exchange or IVIg. IS are generally started at the same time to sustain clinical response. Corticosteroids are known to potentially worsen crisis and are started after IVIg or plasma exchange.
During pregnancy, oral pyridostigmine is the first line treatment. If an additional treatment is required, corticosteroid agent is the best choice. Azathioprine and cyclosporine are relatively safe in case of corticosteroid contraindication. IVIg can be used when a prompt response is sought. Delivery should be preferentially performed in a hospital with a neonatal critical care unit.
Adjunct therapies
Thymectomy is required in case of thymic abnormalities (thymoma or hyperplastic thymus). Histological classification (from benign thymoma to carcinoma) guides further treatment, as malignant tumors require radiotherapy and/or chemotherapy. Radiotherapy alone could be considered in elderly or multimorbid patients.
Some authors recommend thymectomy, even in the absence of thymoma, in patients aged less than 40 years or poor responders to medical treatment.
Possible prevention strategies
Many drugs are contraindicated in MG, such as curare, aminosides, cyclines, fluoroquinolone, beta-blocker, D-penicillamine, and magnesium. Relative contraindications concern benzodiazepines, lithium and neuroleptics.
Download images for this case
Final outcome
At initial diagnosis when Elsa was pregnant, her disease was classified MGFA IIIa. After introduction of pyridostigmine, she rapidly reached minimal manifestations.
Then, she presented an MG crisis after the birth of Elvis. This exacerbation necessitated hospitalization in intensive care unit but no intubation was required. IVIg were administered in emergency and both azathioprine and corticosteroids were started. This treatment led to amelioration (an improvement in condition). After obtaining remission, thymectomy was performed. Histological classification concluded follicular hyperplasia without malignancy. No complemental treatment was therefore required.
After 1 year of follow up, azathioprine is continued and Elsa is considered in complete stable remission.
Download images for this case
References
Deenen JCW, Horlings CGC, Verschuuren JJGM et al. The epidemiology of neuromuscular disorders: a comprehensive overview of the literature. J Neuromuscul Dis 2015;2:73–85
Link to Abstract
Engstrom JW. Myasthenia Gravis: diagnostic mimics. Semin Neurol 2004;24:141-7
Link to Abstract
Evoli A, Tonali PA, Monaco M et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia Gravis. Brain 2003;10:2304-11
Link to Abstract
Gilhus NE. Myasthenia Gravis. N Engl J Med 2016;375:2570-81
Link to Abstract
Gomez AM, Van Den Broeck J, Vrolix K et al. Antibody effector mechanisms in myasthenia Gravis-pathogenesis at the neuromuscular junction. Autoimmunity 2010;43:353-70
Link to Abstract
Gradolatto A, Nazzal D, Truffault F et al. Both Treg cells and Tconv cells are defective in the Myasthenia Gravis thymus: roles of IL-17 and TNF-α. J Autoimmun 2014;52:53-63
Link to Abstract
Jaretzki A III, Barohn RB, Ernstoff RM et al. Myasthenia Gravis : recommendations for clinical research standards : Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 2000;55:16-2
Link to Abstract
Pevzner A, Schoser B, Peters K et al. Anti-LRP4 autoantibodies in AChR- and antiMuSK- antibody negative myasthenia Gravis. J Neurol 2011;259:427-3
Link to Abstract
Sanders DB, Wolfe GI, Benatar M et al. International consensus guidance for management of myasthenia Gravis: Executive summary. Neurology 2016;87:419-25
Link to Abstract
Weiss JM, Cufi P, Le Panse R, Berrrih-Aknin S. The thymus in autoimmune Myasthenia Gravis: Paradigm for a tertiary lymphoid organ. Rev Neurol (Paris). 2013;169:640-9
Link to Abstract
Wolfe GI, Kaminski HJ, Cutter GR. Randomized trial of thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22 (Comment in 2006;375:2006-7)
Link to Abstract
Download images for this case
Evaluation – Questions & Answers
Why was MG the final diagnosis?
Elsa initially presented diplopia, droopy eyelids, asthenia and peripheral weakness.
- Brain tumor was excluded because there were no usual signs of intracranial hypertension: vomiting, blurred vision, headaches.
- Multiple sclerosis (MS) was ruled out due to the progressive onset of symptoms. MS starting before the age of 40 years is recurrent-remittent, e.g. symptoms appear over hours or days, not over weeks.
- Vertebro-basilar insufficiency usually affects patients over the age of 60 years and involves a positional trigger.
- Chronic progressive ophtalmoplegia is generally isolated, without general signs.
- The absence of goiter and normal level of TSH eliminated Graves’ disease (autoimmune hyperthyroidism).
- Presence of diplopia and fluctuations eliminate myositis.
Myasthenic syndromes may be caused by Myasthenia Gravis, Lambert-Eaton syndrome and botulism. They should be considered when only motor (not sensitive) symptoms are present. Yet, between the three myasthenic syndromes, botulism was eliminated by the absence of an evocative context (suspect can of food, botulinum toxin treatment). Lambert-Eaton syndrome is characterized by prominent proximal muscle weakness while ocular signs are absent or mild. Typically, this is a paraneoplastic syndrome associated with anaplasic small cell lung carcinoma. Elsa was too young for this type of cancer and a non-smoker.
Finally, electromyogram was highly evocative and the presence of anti-AChR confirmed the diagnosis of MG.
What are the arguments in favour of the autoimmune nature of this disease?
- Elsa’s sister has autoimmune (type 1) diabetes.
- Autoantibodies are present.
- Patient responds to corticosteroids.
Typically, the autoimmune nature of a disease is supported by the Rose and Witebsky criteria:
- Presence of autoantibodies or autoreactive T cells (here, anti-AChR autoantibodies)
- The autoantigen is identified (here, AChR)
- Immunization with autoantigen induces disease in animals (immunization of rabbit/rodents with AChR induces anti-AChR antibodies and provokes a myasthenic syndrome)
- Passive transfer of autoantibodies from patient to animal induces disease (passive transfer of IgG from MG patients induces a myasthenic syndrome)
- Efficacy of immunosuppression (Elsa responded to corticosteroids + IVIg + azathioprine)
What happened to Elvis?
Elvis developed neonatal myasthenia; due to the transplacental transfer of pathogenic IgG from his mother during gestation. Symptoms of myasthenia disappeared with IgG after 2 to 3 weeks. Elvis is not at risk of MG himself. Pyridostigmine can be prescribed symptomatically during this period.
How do you explain the thoracic mass?
The thoracic mass evidenced by tomography corresponds to a thymic mass. In the context of MG, a thymic mass corresponds to thymic hyperplasia or tumor. Thymic tumor may range from benign thymoma to carcinoma.
Do you know another autoimmune disease which involves pathogenic autoantibodies acting on a receptor?
Graves’ disease is another form of autoimmune disease in which autoantibodies have a direct pathogenic effect on the function of their target, i.e. antibodies directed against thyroid-stimulating hormone (TSH) receptor. In normal conditions, this receptor stimulates the production of thyroid hormones (T3 and T4) in response to TSH. Anti-TSH-receptor autoantibodies bind the receptor and mediate a positive signal, causing hyper-production of thyroid hormones that result in goiter and hyperthyroidism.
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
Download images for this case










