- Patient Presentation
- History
- Differential Diagnosis
- Examinations
- Investigations
- Discussion
- Treatment
- Final Outcome
- References
- Evaluation - Questions & Answers
- MCQ
Patient Presentation
A 34 year old female patient was brought into the outpatient clinic by her family who were concerned about her progressive forgetfulness and inability to take care of herself. She also appeared to have some lower limb weakness that had developed progressively over the course of two weeks.
Acknowledgment
This case study was kindly provided by Dr D. Veliotes and Dr A Richardson from the Department of Neurology, Charlotte Maxeke Johannesburg Academic Hospital.
History
For six weeks prior to presentation, the family had noted that the patient took longer to complete daily tasks and had become progressively more forgetful. She was unable to follow conversations with her family, appearing confused or unable to concentrate. Earlier that week she had got lost walking home from her taxi stop and had been unable to calculate the change when making a small purchase at the local shop. She had not been to work for the last month as she was unable to plan the commute and do her job as a housekeeper in a nearby suburb.
It was also noted that she had had chronic diarrhoea for the last 6 weeks, passing watery stools several times a day. Additionally she complained of headaches and worsening weakness in her legs, but not her arms. She also denied any difficulties with bowel or bladder control.
Past medical history
The patient was diagnosed with HIV two months before with a CD4 count of 49 cells mm3 and had been started on ARVs (Lamivudine, Zidovudine and Efavirenz). The remainder of her medical history was non-contributory with no history of diabetes, epilepsy, asthma, hypertension, rheumatic heart disease, tuberculosis, previous limb weakness or strokes. She also had no known allergies and was not taking any other medication. She had not undergone any previous surgery. She had no significant family history, no travel history and no history of smoking or alcohol use.
Differential Diagnosis
- Schizophreniform disorder
- HIV associated dementia
- Toxoplasmosis
- Cryptococcal meningitis
- Neurosyphilis
- Cytomegalovirus
- Cerebrovascular accident
Examinations
Vitals
Temperature 38°C
Blood pressure 101/76
Heart rate 121 beats per minute
Respiratory rate 22 breath per minute
HGT 6.1
General
The patient appeared wasted, with significant oral thrush, generalized lymphadenopathy and mild dehydration.
Respiratory
Crackles were present in the right lower lobe
Oxygen saturations were normal, 95%
Patient was not in respiratory distress or failure.
Cardiovascular
No abnormalities detected with normal heart sounds, no bruits, no murmurs and no signs of failure.
Abdomen
Not distended
Soft and non-tender with no hepato-splenomegaly
Bowel sounds present
Neurological Exam
The patient had a GCS of 15/15, but on closer examination was cognitively blunted. She was apathetic, inattentive and unable to follow conversation fully.
On the mini mental status exam (MMSE) she scored 17/30, losing points on attention and calculation, recall, language and complex commands. No frontal lobe release signs were elicited, indicating that primitive reflexes (grasp/pout/suck/glabellar) were absent. Cranial nerves were all normal, with pupils equal and reactive to light, and normal fundi. No loss of smooth pursuit or saccadic eye movements. On motor examination, she had normal power, tone and reflexes in the upper limbs. Lower limbs had reduced power 4/5 bilaterally, normal tone and normal reflexes except at the ankles which had reduced reflexes 1/4 bilaterally. Normal sensation globally. No evidence of cerebellar dysfunction. No limb incoordination. No ataxia, but slow gait noted.
Investigations
| Examination | Value |
|---|---|
| WBC | 9.49 |
| HB | 8.3000000000000007 |
| MCV | 93.9 |
| Platelets | 274 |
| ESR | 155 |
| T4 | 12 |
| TSH | 1.64 |
| B12 | >734 |
| Alb | 17 |
| Corrected Ca | 2.2200000000000002 |
| Mg | 0.95 |
| PO4 | 0.5 |
| RPR | Negative |
| TPHA | Negative |
| NA (over 5 days) | 112 - 124 – 132 |
| K (over 5 days) | 4.2 - 3.5 - 3.2 |
| CL (over 5 days) | 76 - 90 – 106 |
| C02 (over 5 days) | 20 - 17 – 13 |
| Urea (over 5 days) | 8.5 - 4.5 - 1.0 |
| Creatinine (over 5 days) | 84 - 61 – 39 |
| Lumbar puncture | Poly 0 |
| Lymphocy 0 | |
| Eryth 0 | |
| Protein 0.17 | |
| Gluc 2.0 | |
| Chloride 117 | |
| Cytology - Negative | |
| CSF latex agglutination test - Negative | |
| India Ink - Negative | |
| No bacterial growth | |
| Sputum | AFB - Negative x2 |
| Stool culture | Cryptosporidium + |
| Urine dipstix | 2+Leukocytes |
| HIV Elisa | Positive |
CXR:
Consolidation in right lung base, no evidence of multilobar involvement, no evidence of pleural effusion.
CT brain:
Age inappropriate involution
Small areas of gliosis in left parietal lobe indicative of old infarct
Nerve conductions:
Normal velocities and amplitudes
International HIV dementia scale:
- Motor speed 2/4
- Psychomotor speed 3/4
- Memory recall 1/4
Total score: 6/12
Interpretation: there is a strong likelihood of dementia, this requires further examination including MRI, LP, HIV test if status unknown and consultation with neurologist.
Management of HAD and important things not to miss:
| INTERNATIONAL HIV DEMENTIA SCALE (IHDS) | |
|---|---|
| International HIV Dementia Scale (IHDS) has been validated to detect mild to severe neurocognitive impairment in HIV-infected patients | |
| Memory-Registration – Give four words to recall (dog, hat, bean, red) – 1 second to say each. Then ask the patient to repeat all four words after you have said them. Repeat words if the patient does not recall them all immediately. Tell the patient you will ask for recall of the words again a bit later. | |
| 1 | Motor Speed: Have the patient tap the first two fingers of the non-dominant hand as widely and as quickly as possible. |
| 4 = 15 in 5 seconds | |
| 3 = 11-14 in 5 seconds | |
| 2 = 7-10 in 5 seconds | |
| 1 = 3-6 in 5 seconds | |
| 0 = 0-2 in 5 seconds | |
| 2 | Psychomotor Speed: Have the patient perform the following movements with the non-dominant hand as quickly as possible: |
| 1. Make a fist and rest it on a flat surface. | |
| 2. Put hand flat on surface with palm down. | |
| 3. Put hand perpendicular to flat surface on the side of the 5th digit. Demonstrate and have patient perform twice for practice. | |
| 4 = 4 sequences in 10 seconds | |
| 3 = 3 sequences in 10 seconds | |
| 2 = 2 sequences in 10 seconds | |
| 1 = 1 sequence in 10 seconds | |
| 0 = unable to perform | |
| 3 | Memory-Recall: Ask the patient to recall the four words. For words not recalled, prompt with a semantic clue as follows: animal (dog); piece of clothing (hat); vegetable (bean); color (red). |
| Give 1 point for each word spontaneously recalled. | |
| Give 0.5 points for each correct answer after prompting | |
| Maximum – 4 points. | |
| Total International HIV Dementia Scale Score: This is the sum of the scores on items 1-3. The maximum possible score is 12 points. | |
| A patient with a score of ≤ 10 should be evaluated further for possible dementia. In this case, consider CNS nuclear magnetic resonance imaging, lumbar puncture including CSF HIV RNA measurement, and consultation with neurologist or neuropsychologist. |
Discussion
HIV and the brain
Since the widespread use of highly active antiretroviral therapy (HAART), the incidence, but not the prevalence, of HIV- associated neurocognitive disorders (HAND) has decreased. These disorders incorporate mild impairment of higher cognitive and cortical functions including minor cognitive motor disorders and asymptomatic neurocognitive impairment characterized by cognitive, motor, and behavioral abnormalities. Classification is based on patient performance in areas of neurological and behavioral functioning and neuropsychological testing. The outcomes are on a spectrum with the most severe form of HAND being HIV associated dementia (HAD) also known as AIDS dementia. HAD manifests as a subcortical dementia characterized by psychomotor slowing, changes in mood and anxiety levels and deficits in memory, abstraction, information processing, verbal fluency, decision-making, and attention. As this collection of symptoms would suggest, the brain regions most commonly damaged in HAD are the basal ganglia, deep white matter, hippocampus, and cerebral cortex. HAD has also been noted to include deficits in olfaction which although a surprise finding allows for reliable measurement of the severity, onset, and progression of the disease using well established olfactory tests. While 40% of HIV positive patients on HAART continue to suffer from HAND, the incidence of HAD has dramatically decreased. However its prevalence is increasing, due in part to the increasing life expectancy of individuals with HIV.
How does this happen?
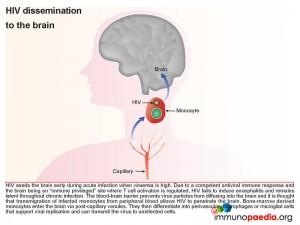
First we look at HIV dissemination to the brain
The blood-brain barrier (BBB), which is comprised of a basement membrane and tight-junctions between capillary endothelial cells prevents large molecules, or pathogens, from diffusing into the brain, such as HIV. However bone marrow derived monocytes are able to enter the brain, where they then differentiate into perivascular macrophages and microglial cells. It is thus thought that because HIV is capable of infecting monocytes at a low level, during the high viraemia during acute infection, a small number of monocytes in the peripheral blood will become infected and some of these will enter the brain by transmigration across the BBB (known as diapedesis) that takes place in the post-capillary venules. However, due to a competent antiviral immune response and the brain being an “immune privileged” site where T cell activation is regulated, viral replication is controlled and no encephalitis is induced and HIV remains latent.
Although infected CD4+ T cells may also infiltrate the brain, the transmigration of infected monocytes from the systemic circulation is the proposed mechanism by which HIV initially penetrates the brain shortly after transmission.
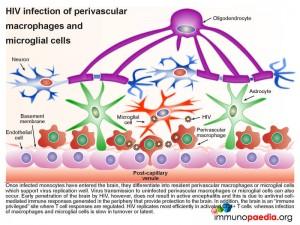
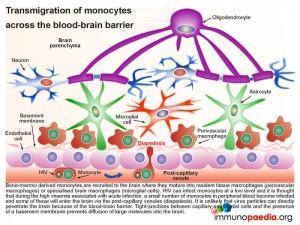 In more detail, once the infected monocytes enter the brain, they differentiate into resident perivascular macrophages or specialized brain macrophages known as microglial cells. These cells then support virus replication and can transmit virus to uninfected perivascular macrophages and microglial cells. Penetration of the brain by HIV during early infection, however, does not progress to viral encephalitis, due to adequate control of viral replication by HIV-specific CD8+ cytotoxic T cells and CD4+ helper T cells that are recruited to the brain from the periphery. HIV remains latent within perivascular macrophages or microglial cells throughout the asymptomatic period. This is in part, because the brain is an “immune privileged” site where T cell activation and inflammatory responses are regulated to avoid immune-mediated damage to neurons and glial cells. HIV propagates more efficiently in activated CD4 T cells than in macrophages or microglial cells. The turnover of virus in monocyte-derived cells is slow and non-cytopathic, hence, these cells serve as reservoirs of HIV during the chronic stage of disease. It can be shown by phenotypic analysis that strains of HIV isolated from the brain are adapted to monocyte-derived cells and preferentially use the CCR5 co-receptor for entry.
In more detail, once the infected monocytes enter the brain, they differentiate into resident perivascular macrophages or specialized brain macrophages known as microglial cells. These cells then support virus replication and can transmit virus to uninfected perivascular macrophages and microglial cells. Penetration of the brain by HIV during early infection, however, does not progress to viral encephalitis, due to adequate control of viral replication by HIV-specific CD8+ cytotoxic T cells and CD4+ helper T cells that are recruited to the brain from the periphery. HIV remains latent within perivascular macrophages or microglial cells throughout the asymptomatic period. This is in part, because the brain is an “immune privileged” site where T cell activation and inflammatory responses are regulated to avoid immune-mediated damage to neurons and glial cells. HIV propagates more efficiently in activated CD4 T cells than in macrophages or microglial cells. The turnover of virus in monocyte-derived cells is slow and non-cytopathic, hence, these cells serve as reservoirs of HIV during the chronic stage of disease. It can be shown by phenotypic analysis that strains of HIV isolated from the brain are adapted to monocyte-derived cells and preferentially use the CCR5 co-receptor for entry.
Disease progression
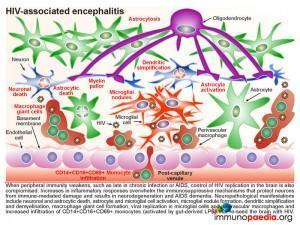
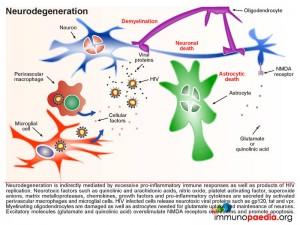
In our current case, it was likely that as her disease progressed, there was an eventual lack of immune control of HIV infection in the periphery. During late stage infection or AIDS, latent HIV replication is activated, including in the brain. HIV replication in the brain resulted in an increase in inflammatory cytokines that overwhelmed the immune regulatory mechanisms and lead to damage of neurons resulting in the presenting HAD. Neuropathological manifestations of HIV replication in the brain includes: a) death of neurons and astrocytes; b) activation of astrocytes and microglial cells; c) formation of microglial nodules, c) dendritic simplification d) demyelination; e) formation of macrophage giant cells (fusion of HIV-infected macrophages); f) viral replication in microglial cells and perivascular macrophages and g) increased infiltration of monocytes. Of note, during this stage a predominance of CD14+CD16+ and/or CD69+ monocytes are recruited to the brain. These cells are most likely expanded during HIV infection as a result of translocation of gram negative bacterial products, such as lipopolysaccharide (LPS), from the gut. The binding of LPS to toll-like receptor 4, which uses CD14 as a co-factor, promotes activation and expansion of monocytes, which in turn are capable of trafficking to the brain and contributing to inflammation as well as re-seeding the brain with virus. It is postulated that “leaky” gut in HIV infection may in fact trigger the onset of HIV encephalitis.
Neurons, astrocytes and oligodendrocytes are not considered targets for HIV. Neurodegeneration is thought to be indirectly caused by inflammatory mediators released from activated macrophages and microglial cells during excessive inflammatory responses as well as by neurotoxic viral products, such as gp120, tat and vpr, released from infected cells. Astrocytes are also damaged leading to reduced glutamate reuptake and diminished maintenance of neurons. Excess glutamate overstimulates the NMDA receptors on neurons, which controls calcium influx and can promote apoptosis. Damage to oligodendrocytes affects the myelin sheath necessary for nerve signal transduction.
Protective mechanisms for the brain – the brain as an immune privileged site
Immune privileged sites include the eye, the brain, placenta and testis and are so-called because of mechanisms of immune tolerance, which protect these vital tissues from immune-mediated damage. In our case study, uncontrolled HIV in the brain at the late stage of disease, resulted in activation of inflammatory mediators resulting in neurotoxicity and the likely irreparable damage to neurons. Therefore controlling immune-mediated damage is extremely important within immune privileged sites, ie the brain. Under normal circumstances, this is achieved by both physiological barriers that control antigens and immune cell movement and immune regulatory mechanisms, which control T cell responses and activation of antigen-presenting cells. More detailed explanations with corresponding graphics follow below.
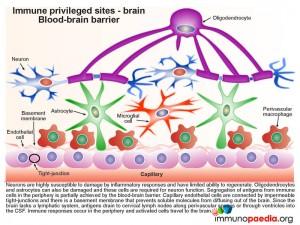
The blood-brain barrier (BBB)
Neurons are highly susceptible to damage by inflammatory responses and have limited ability to regenerate. Oligodendrocytes and astrocytes, required for neuron function, are also vulnerable to immune-mediated damage. The BBB thus provides a physiological barrier comprised of capillary endothelial cells connected by impermeable tight-junctions and a basement membrane that restricts the passage of immune cells into the brain and prevents brain-derived antigens from diffusing into the peripheral circulation
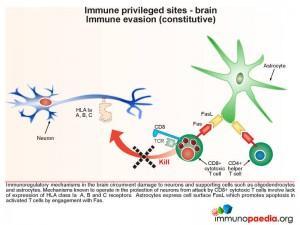
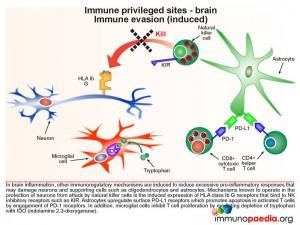
Immune evasion (constitutive type)
Immunoregulatory mechanisms in the brain circumvent damage to neurons and supporting cells such as oligodendrocytes and astrocytes. This includes a constitutive lack of expression of MHC class I molecules (known as HLA class Ia A, B and C), and hence the inability to be attacked by CD8+ cytotoxic T lymphocytes (CTL). Astrocytes express cell surface FasL to promote apoptosis in activated T cells by engagement with surface expressed Fas.
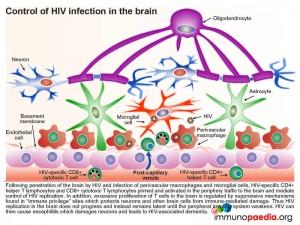
During an inflammatory response in the brain, some immunoregulatory mechanisms are induced in order to limit the extent of immune damage.
One of the mechanisms known to protect neurons from attack by natural killer cells is the induced expression of HLA class Ib G receptors that bind to NK inhibitory receptors, such as KIR (killer immunoglobulin-like receptors). This results in the inhibition of NK cell function which is usually triggered by the lack of detection of HLA class I A, B and C receptors. Another inducible inhibitory mechanism, is the ability of astrocytes to promote apoptosis in activated T cells by upregulating surface PD-L1 receptors which can engage with PD-1 on T cells. Microglial cells can also inhibit T cell proliferation by mediating depletion of the essential amino-acid tryptophan with expression of indolamine 2,3-dioxygenase (IDO) enzymes.
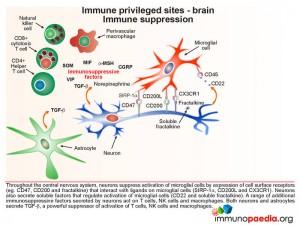
Microglial cells are abundant in the brain and serve to phagocytose apoptotic cell debris and harmful substances in the extracellular fluid. They can become activated and potentially damage neurons when there is an infection. Throughout the central nervous system, neurons suppress activation of microglial cells by expression of cell surface receptors that interact with ligands on microglial cells and soluble factors that regulate activation. They also secrete a range of additional immunosuppressive factors which act on T cells, NK cells and macrophages.
In a situation such as HAD (and in this Case Study) the inflammatory responses that result from uncontrolled HIV replication, overwhelm these processes that preserve the integrity of the brain compartment.
Download images for this case
Treatment
On admission:
- IVI rehydration fluids to correct electrolyte abnormalities
- IVI Augmentin for treatment of lower respiratory tract infection
- Clexane daily for DVT prophlaxis
Continued treatment:
- Continue with HAART, also include bactrim and multi vitamins
- Physiotherapy
Currently, HAART is the cornerstone of treatment for HIV-related cognitive disorders as defended by multiple randomized, placebo-controlled trials. Strategies for Management of Anti-Retroviral Therapy 2006 (SMART), a multicenter international trial and one of the largest HIV/AIDS treatment trials conducted, shows benefit of continuous treatment compared with episodic treatment. Furthermore, early and continuous viral suppression with HAART is associated with improved performance on neuropsychological testing.
Download images for this case
Final Outcome
The patients power in the lower limbs improved significantly over the hospital admission, as did the patient’s electrolytes, see day 0, 2 and 5 of hospital stay.
Early HIV associated dementia (HAD) also known as AIDS dementia complex (ADC), was assessed using the international HIV dementia scale as described under “investigations”
The patient was continued on her HAART regimen and has received ongoing adherence counselling.
She participates in regular occupational therapy and psychiatric therapy and her ARV regimen will continue to be monitored on an outpatient basis at the ARV clinic
The patient was stepped down to a secondary hospital for observation prior to discharge home.
Download images for this case
References
Sadagopal S et al. (2008). Enhancement of human immunodeficiency virus (HIV)-specific CD8+ T cells in cerebrospinal fluid compared to those in blood among antiretroviral therapy-naive HIV-positive subjects. J Virol. Nov;82(21):10418-28.
Niederkorn JY. (2006). See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. Apr;7(4):354-9.
Galea I et al. (2007). What is immune privilege (not)? Trends Immunol. Jan;28(1):12-8
Download images for this case
Evaluation – Questions & Answers
What is the diagnosis?
What is the blood brain barrier (BBB)?
During what stage of HIV infection does the virus seed to the brain?
Assuming the blood brain barrier is intact, how does HIV gain access to the brain?
It is thought that because HIV is capable of infecting monocytes at a low level, during the high viraemia associated with acute infection, a small number of monocytes in the peripheral blood will become infected and some of these cells are recruited to the brain where they differentiate into microglial cells or perivascular macrophages. These cells can “seed” the brain with HIV because they are capable of crossing the blood-brain barrier by diapedesis in the post-capillary venules.
What makes the brain an immune privileged site?
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
Download images for this case