- Patient Presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final Outcome
- References
- Evaluation - Questions & Answers
- MCQ
Patient Presentation
A 5 ½ year old male child is referred from an HIV treatment clinic to the paediatric department at his local hospital because he was noted to be severely anaemic at his last visit.
History
TM is a 5½ year old boy who was diagnosed as HIV positive at his local clinic and started on HAART. He was initiated on a regimen of d4T, 3TC and EFV. Three weeks after starting treatment, his family relocated and he was transferred to an HIV treatment clinic. At that time his CD4 count and viral load were checked with the following results:
- CD4= 39 (1.74%)
- Viral load = <25 copies
His treatment remained unchanged.
One week later he presented to the clinic, with signs and symptoms of severe anaemia. His Hb was checked, found to be 1.2 g/l and he was immediately admitted to the paediatric ward.
Past Medical History
- Nothing of significance
Past Surgical History
- Nil
Vaccination History
- Up to date
Developmental History
- All milestones have been met
- Growth parameters are within normal limits
Family History
- Father passed away when TM was age 2, cause unknown
- Mother is HIV +, on HAART
Allergies
- None known
Medication
- d4T, 3TC and EFV
- Multivitamins
Travel History
- Recent move from Eastern Cape. No other travel noted
Social History
- Lives with mother, aunt and two cousins in an informal settlement, with limited amenities.
Differential Diagnosis
- Drug toxicity
- Nutritional deficiency
- Anaemia of chronic disease
- Helminth infection
- Parvovirus infection
- Other viral infection
- Underlying malignancy
Examination
Appearance
- Ill looking child, pale and lethargic but co-operative
- GCS 15/15
Vitals
- Temperature: 37.0°C
- Blood pressure: 90/58
- Heart rate: 110
- Respiratory rate: 20
General
- Extreme pallor
- No jaundice
- Pale, cold peripheries
Head and Neck
- Conjunctival pallor
- Pale oral mucus membranes
Chest
- Rapid shallow breathing
- Chest clear
Cardiovascular
- Tachycardia
Abdomen
- Not distended
- Soft, no generalised tenderness
- No organomegally
- Bowel sounds present
- No abnormalities detected
Neurological
- Normal level of consciousness
- Gait normal
- Power 5/5 globally
- Tone normal globally
- Reflexes 2/4 for both upper and lower limbs
- No signs of peripheral neuropathy
Dermatological
- No skin rashes noted on examination
Investigations
| Test | On admission | After 6 days | After 9 days | 6 weeks | 1 month later re-admitted | 4 days later | 1 week later discharged | ref ranges |
|---|---|---|---|---|---|---|---|---|
| discharged | ||||||||
| Transfused | Transfused + IVIG | Transfused + IVIG | ||||||
| WBC | 5.53 | 6.19 | 7.62 | 7.14 | 17.16 | 8.449 | 8.58 | (4-12 x109/L) |
| HB | 1.2 | 8.300 | 5.5 | 10.199 | 3.7 | 9.4 | 10.199 | (12.1-15.2 g/L) |
| RCC | 0.46 | 3.03 | 2.14 | 3.58 | 1.47 | 3.52 | 3.7 | 4.05- 5.15 x1012/L |
| HCT | 0.04 | 0.24 | 0.18 | 0.32 | 0.11 | 0.31 | 0.32 | 0.353- 0.428 |
| MCV | 90.6 | 80.599 | 84.4 | 89.2 | 76 | 86.7 | 87 | (77-91) |
| MCH | 25.2 | 27.5 | 25.8 | 28.5 | 24.9 | 26.6 | 27 | 25.8- 31.7 |
| MCHC | 27.8 | 34.1 | 30.5 | 32 | 32.1 | 30.7 | 31.5 | 33.0- 35.1 |
| Platelets | 289 | 225 | 1085 | 523 | 424 | 517 | 532 | (140-450 x109/L) |
| Retic. count | 0.11 | 6.86 | 7.89 | |||||
| Absolute retic. count | 2E-3 | 0.2409 | 0.2939 | |||||
| CD4+ | 39 (1.74%) | 441 | ||||||
| Viral load | (Copies per ml) | |||||||
| Parvovirus IgG | Neg | pos | ||||||
| Parvovirus IgM | Neg | pos | ||||||
| Coombs | Negative |
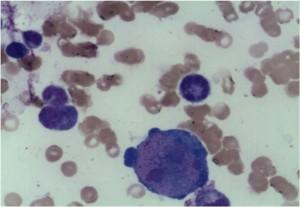
Bone marrow biopsy report:
Erythropoesis was shown to be hypocellular with occasional giant pronormoblasts. Erythroid precursors were virtually absent in the bone marrow.
Microscopy shows that the erythroid series is profoundly hypocellular and consists predominantly of bizarre, giant pronormoblasts with no maturation beyond this stage. No other erythroid elements present.
Microscopy shows that the erythroid series is profoundly hypocellular and consists predominantly of bizarre, giant pronormoblasts with no maturation beyond this stage. No other erythroid elements present.
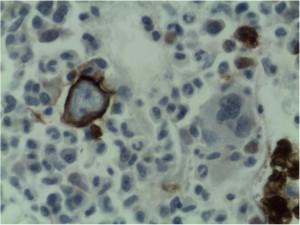 Final comment from bone marrow and trephine report:
Final comment from bone marrow and trephine report:
The profound anaemia, poor reticulocyte response and virtual absence of erythroid precursors in the bone marrow are in keeping with the diagnosis of Pure Red Cell Aplasia. Morphological features are in keeping with Parvovirus B19 infection (i.e. giant pronormoblasts and the absence of mature erythroid precursors is pathognomonic of parvovirus B19 infection.) However if serological and molecular testing is negative other viral infections, drugs, underlying malignancy and primary immune mechanisms should also be considered as causative factors. HIV itself may rarely cause this condition.
The dysplastic features noted in the megakaryocytic lineage are due to HIV infection.
Discussion
Background
In our case study, the patient was confirmed as infected with Parvovirus B19, which is a single-stranded non-enveloped DNA virus. Although parvoviruses are well known to cause disease in animals, parvovirus B19 is a strictly human pathogen. A commonly encountered manifestation of parvovirus B19 is childhood exanthem erythema infectiosum or Fifth disease, also known as slapped cheek syndrome. Aside from this characteristic rash, which is seen in both paediatric and adult patients, parvovirus B19 also causes fever, headaches, sore throat, arthralgias and anaemia. In healthy individuals, this anaemia is mild and of short duration, but in individuals with weakened immune systems the resulting anaemia can be severe and long-lasting.
Most people with Fifth disease remain asymptomatic at the time of infection. Serological studies in adults have identified parvovirus B19 antibodies in 50-60% of the population, indicating infection is common and most likely occurs in childhood.
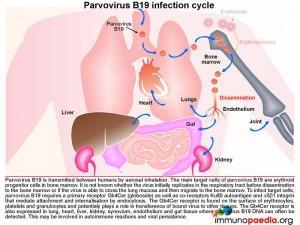 Transmission of Parvovirus B19
Transmission of Parvovirus B19
Parvovirus B19 is transmitted between humans by aerosol inhalation. The main target cells of the virus are the erythroid progenitor cells located in the bone marrow. It is unknown whether the virus first replicates at a low level in the respiratory tract before disseminating to the bone marrow or if the virus has the ability to cross the lung mucosa and then traffic to the bone marrow. Parvovirus B19 requires a primary receptor Gb4Cer (globoside) as well a co-receptors Ku80 autoantigen, and a5b1 integrin for attachment and internalisation by endocytosis. Globoside is expressed on erythrocytes, platelets and granulocytes and may play a role in viral dissemination to other tissues. Globoside is also known to be present in lung, heart, liver, kidney, synovium, endothelium and gastrointestinal tissues and may provide reservoirs of virus since viral DNA can often be detected in these tissues. This may possibly be associated with viral persistence.
Pathophysiology of parvovirus B19
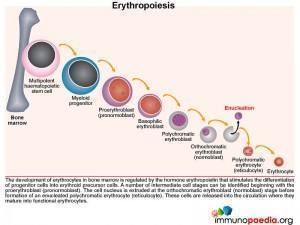
Parvovirus B19 has a unique tropism for human erythroid progenitor cells. Therefore to better understand the disease mechanism we need to first look at erythrocyte development.
The normal development of erythrocytes in the bone marrow is regulated by the hormone erythropoietin that stimulates differentiation of progenitor cells into erythroid precursors. Along this pathway, a number of intermediate cell stages can be identified beginning with the nucleated proerythroblast (pronormoblast). The cell nucleus is then discarded at the orthochromatic erythroblast (normoblast) stage before formation of an enucleated polychromatic erythrocyte commonly known as the reticulocyte. These are released into the circulation where they mature into functional erythrocytes.
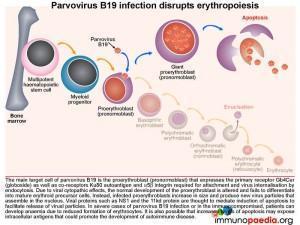 Parvovirus B19 infection and disruption of erythropoesis
Parvovirus B19 infection and disruption of erythropoesis
The main target cell of parvovirus B19 is the proerythroblast (pronormoblast), which expresses the primary receptor Gb4Cer (globoside) as well as the co-receptors Ku80 autoantigen and a5b1 integrin required for attachment and virus internalisation by endocytosis.
Due to viral cytopathic effects the normal development of the proerythroblast is altered and fails to differentiate into mature erythrocyte precursors. Instead infected proerythroblasts increase in size and produce new virus particles that are assembled in the nucleus. Viral proteins such as NS-1 and the 11kd protein are thought to mediate induction of apoptosis of the cell to enable virus release. Increased apoptosis of infected cells are thought to expose cellular antigens at a level that may induce an autoimmune response.
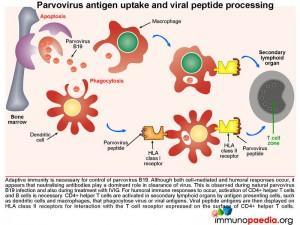 Parvovirus antigen uptake and viral peptide processing
Parvovirus antigen uptake and viral peptide processing
Adaptive immunity is required for control of Parvovirus B19. Although both cell-mediated and humoral responses occur, it seems that neutralising antibodies are capable of resolving infection. For humoral responses to occur, CD4+ helper T cells and B lymphocyte activation is necessary. Naïve CD4+ helper T cells are activated in secondary lymphoid organs (for eg. lymph nodes) by antigen presenting cells such as dendritic cells, that phagocytose virions or viral antigens and express immunogenic peptides on HLA class II receptors for interaction with T cell receptors expressed on CD4+ T cells.
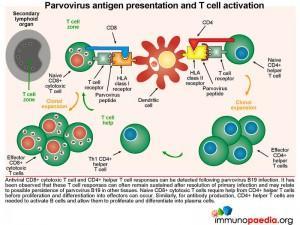 Parvovirus antigen presentation and T cell activation
Parvovirus antigen presentation and T cell activation
Both CD8+ cytotoxic T cell and CD4+ helper T cell responses can be detected following parvovirus B19 infection. It has been observed that these T cell responses can often remain sustained after resolution of infection, which may reflect possible persistence of virus in other tissues such as lung, heart, liver, kidney, synovium, endothelium and gut. For B cells to produce antibodies to parvovirus B19, virus-specific CD4+ T lymphocytes are required to help B cells, through CD40-CD40L interactions and parvovirus B19 peptides bound to the HLA-class II molecules. In our patient, who was HIV-infected, the resulting impairment of CD4+ T cells also likely results in inadequate help for B cells to make these specific antibodies.
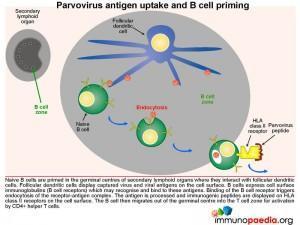 Parvovirus antigen uptake and B cell priming
Parvovirus antigen uptake and B cell priming
Naive B cells are primed in the germinal centres of secondary lymphoid organs where they interact with follicular dendritic cells. Follicular dendritic cells display captured virus and viral antigens on the cell surface. B cells express cell surface
immunoglobulins (B cell receptors) which recognise and bind to these antigens. Binding of the B cell receptor triggers endocytosis of the receptor-antigen complex. The antigen is processed and antigenic peptides are displayed on HLA class II receptors. The B cell then migrates out of the germinal centre into the T cell zone for activation by CD4+ helper T cells.
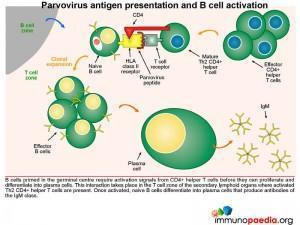
Parvovirus antigen presentation and B cell activation
B cells primed in the germinal centre require activation signals from CD4+ helper T cells before they can proliferate and differentiate into plasma cells. This interaction takes place in the T cell zone of the secondary lymphoid organs where virus-specific CD4+ T cells that have previously been activated by dendritic cells. Newly activated plasma cells initially produce IgM.
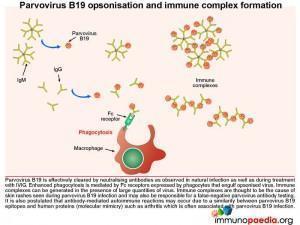 Parvovirus B19 opsonisation and immune complex formation
Parvovirus B19 opsonisation and immune complex formation
In a healthy individual, parvovirus B19 is effectively cleared by neutralising antibodies and in our case study, treatment with intravenous immunoglobulin (IVIG) also results in viral clearance. How does this work? IVIG stimulates enhanced phagocytosis by macrophages and is mediated by Fc receptors on the surface of cells that interact with the Fc portion of the IgG. The IVIG coats the virus, known as opsonisation, and the Fc region of the antibody then binds to the Fc receptor that stimulates the macrophage to engulf the opsonised virus. Immune complexes can also develop in the presence of large quantities of virus and these are thought to be the cause of the skin rashes observed during parvovirus B19 infection (eg. Fifth disease). Importantly, the antigen-antibody immune complexes are likely responsible for a false-negative result in parvovirus antibody testing – as there is no longer free antibody to be detected.
It is also postulated that antibody-mediated autoimmune reactions may occur due to a similarity between parvovirus B19 epitopes and human proteins (a process of molecular mimicry) such as seen in acute and chronic arthritis, which is often associated with this infection.
In immunocompromised individuals, particularly those who are HIV-infected with low CD4 counts and not on treatment, the necessary antibody responses for controlling parvovirus B19 replication may be inadequate due to the lack of CD4+ T cell help. Here we explain how HIV compromises CD4 help during antigen presentation and B cell activation that leads to parvovirus B19-specific antibodies.
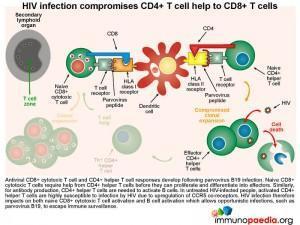 HIV infection compromises CD4+ T cell help
HIV infection compromises CD4+ T cell help
Both virus-specific CD8+ cytotoxic T cell and CD4+ helper T cell responses can be detected following parvovirus B19 infection. Naive CD8+ cytotoxic T cells require help from CD4+ helper T cells before they can proliferate and differentiate into effectors. Similarly, for antibody production, CD4+ helper T cells are needed to provide B cells with the correct signals. During HIV infection, the resulting activated CD4+ T cells become susceptible to HIV due to upregulation of CCR5 co-receptors, resulting in CD4+ T cell dysfunction. Aberrant CD4 functions impact on the ability of both CD8+ T cells and B cells to specifically impart cytotoxic function and IgM and IgG antibody production to parvovirus B19. This scenario results in the host becoming more susceptible to opportunistic infections – and in our case, to parvovirus and the resulting anaemia.
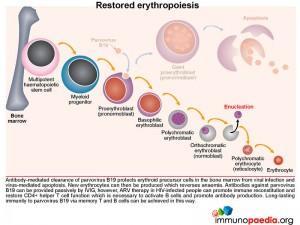
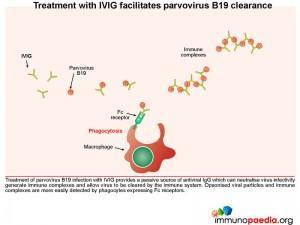
Treatment with IVIG facilitates parvovirus B19 clearance
Treatment of parvovirus B19 infection with IVIG provides a passive source of antiviral IgG, which can neutralize virus infectivity and allow virus to be cleared by the immune system. Opsonised viral particles and immune complexes are more easily detected by phagocytes expressing Fc receptors.
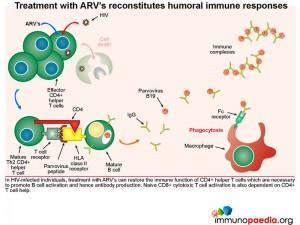
 Treatment with ARV’s reconstitutes humoral immune responses
Treatment with ARV’s reconstitutes humoral immune responses
In HIV-infected individuals, treatment with ARV’s can restore the immune function of CD4+ helper T cells which are necessary to promote B cell activation and hence antibody production. CD8+ cytotoxic T cell responses are also dependent on CD4+ T cell help.
Antibody-mediated clearance of parvovirus B19 protects erythroid precursor cells from viral infection and virus-mediated apoptosis. New erythrocytes can then be produced which reverses anaemia. Anti-viral antibodies can be provided passively by IVIG, but ARV therapy in HIV-infected people can promote immune reconstitution and restore CD4+ helper T cell function necessary to activate B cells and promote antibody production. Long-lasting immunity to parvovirus B19 via memory T and B cells can be achieved in this way.
Download images for this case
Treatment
On admission the patient was transfused, his Hb made a transient recovery and within a week his Hb declined again. Upon re-transfusion and provision of IVIG, his Hb was raised. Six weeks after admission he was discharged and referred to ARV clinic to continue treatment.
Download images for this case
Final Outcome
One month post discharge he was re-admitted for severe anaemia. He was re-transfused and given a 2nd dose IVIG. One week later when his Hb had recovered he was again discharged to continue follow up at the ARV clinic.
Four months later there were no further episodes of anaemia and his CD4 count continued to improve.
Download images for this case
References
Blümel J et al. (2010). Parvovirus B19 – Revised. Transfus Med Hemother. 2010;37(6):339-350. Epub Nov 17. No abstract available.
Norbeck O et al. (2005). Sustained CD8+ T-cell responses induced after acute parvovirus B19 infection in humans. J Virol. Sep;79(18):12117-21.
Cooling LL et al (1995). Multiple glycosphingolipids determine the tissue tropism of parvovirus B19. J Infect Dis. Nov;172(5):1198-205.
Bönsch C et al. (2008). Interaction of parvovirus B19 with human erythrocytes alters virus structure and cell membrane integrity. J Virol. Dec;82(23):11784-91. Epub 2008 Sep 24.
Chen AY et al (2010). The small 11 kDa nonstructural protein of human parvovirus B19 plays a key role in inducing apoptosis during B19 virus infection of primary erythroid progenitor cells. Blood. Feb 4;115(5):1070-80. Epub 2009 Oct 27.
Servant-Delmas A et al (2010). Advances in human B19 erythrovirus biology. J Virol. Oct;84(19):9658-65. Epub Jul 14. Review
Download images for this case
Evaluation – Questions & Answers
What is the diagnosis?
What is the cause of the anaemia?
What is considered a hallmark of parvovirus B19 infection?
What is required for control of Parvovirus B19?
What happens in immunocompromised individuals?
Why does the child no longer require IVIG after the second dose?
What is the cause of negative results in parvovirus antibody testing?
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
Download images for this case