History of Immunoglobulin Molecules
Take a look at our Timeline – History of Immunoglobulin Molecules
Immunoglobulin Structure
- The immunoglobulins are a family of glycoproteins, and based on chemical and structural differences are classified in five distinct classes of molecules called isotypes that are named IgM, IgG, IgA, IgD, and IgE.
- Each immunoglobulin has two small chains (molecular weight of 22 kD) termed light chains (LCs) (Figure 1) and two more chains (55 kD) called heavy chains (HCs).
- Each IgG molecule is composed of two identical LCs and two identical HCs which form a Y-shaped structure (Figure 1).
- The upper two arms perform an antigen-recognition function, which is referred to as the antigen-binding fragment (Fab).
- The lower stem of the molecule displays an effector function and interacts with other components of the immune system such as complement and cell receptors specific for this part of the molecule, which is referred to as the crystallizable fragment (Fc) (Figure 1).
- Another fragment of immunoglobulin, referred to as the Fv fragment, retains the complete antibody-binding sites and consists of the variable regions of both heavy and light chains containing the N-terminal half of the Fab. These fragment have been generated from the use of a DNA recombinant expression system and are used clinically, for example, when treating cancer patients with mouse monoclonal antibodies specific for tumor antigens. However, the efficacy of these monoclonal fragments is diminished since they lack the constant regions essential for many of the effector functions.
- Both light and heavy chains of the immunoglobulin molecule are further divided into regions referred to as variable (V) and constant (C) regions (Figure 1B)
- The variable region is responsible for the highly specific antigen-recognition function of an individual antibody molecule for its antigen (Figure 1C).
- LCs contain two domains, i.e., the variable light (VL) and the constant light (CL); the heavy chains contain one variable heavy (VH) domain and either three or four constant CH domains depending on the immunoglobulin isotype.
- The constant region of the IgG, IgA, and IgD HCs contain three domains and the constant regions of IgM and IgE have an extra domain resulting in four domains.
- In the Y-shaped immunoglobulin molecule is a proline-rich region between the first (CH1) and second (CH2) domains of the HC called the hinge region. This region contains cysteine residues that allow linkage of the HC polypeptides to each other by S-S bonds.
- This region confers flexibility to the molecule by providing mobility of the two upper arms of the molecule, thus enhancing their antigen-binding potential.
- Two immunoglobulin isotypes, IgM and IgE, do not have hinge regions; however, their CH2 regions perform a hinge-like function.
- Immunoglobulin isotypes are named by their HCs (γ, α, μ, δ, and ɛ ) and contain two types of LCs.
- There are two different types of LCs in each of the isotypes, kappa (κ) and lambda (λ).
- In contrast to the kappa isotype, which is of only one type, there are four slightly different constant region sequences of the lambda LC, forming four subclasses (subtypes).
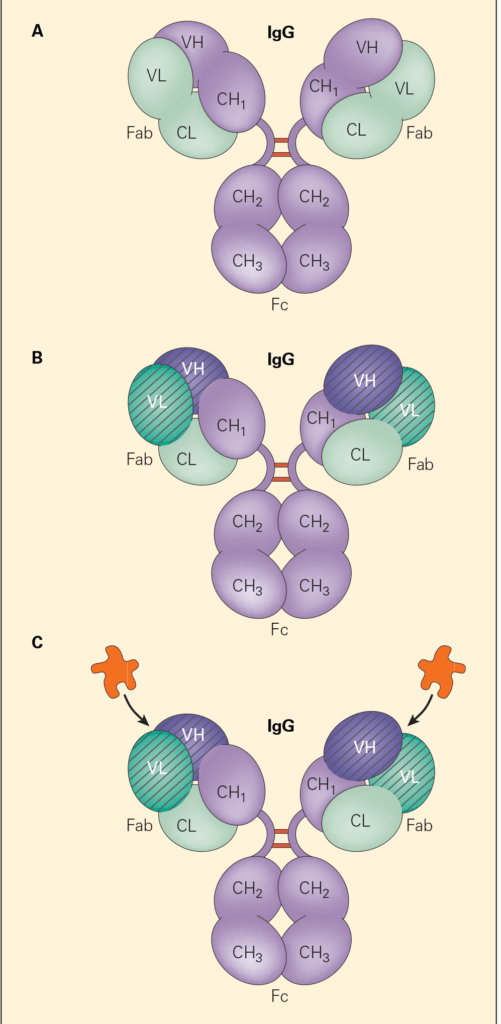
Figure 1. Panel A: A schematic representation of the IgG molecule showing the two light chains and the two heavy chains and the location of interchain disulfide bonds in the hinge region. The amino terminal end is at the top and the carboxyl terminal end is on the bottom. Panel B: The variable regions of the light and heavy chains (i.e., VL and VH, respectively, crosshatched) that, together with the constant regions of the light (CL) and the heavy (CH1), make up the antigen-binding region (Fab) of the molecule. Panel C: The binding of antigen with the antigen-binding region (Fab). [Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].
Biological Functions of Immunoglobulins
- Immunoglobulins can either be found as transmembrane proteins on the surface of the B cell or they can be secreted by the terminal cell of B cell differentiation, i.e., the plasma cell.
- Immunoglobulins function as antibodies and have the property to combine with the antigen (i.e., immunogen) that triggered their production.
- This unique property of recognition, referred to as specificity, is controlled by an amazing assortment of genes that regulate the production of individual parts of the immunoglobulin molecule by determining the primary amino acid sequence of these components.
- Immunoglobulins are specialized molecules that basically provide two functions:
- (1) an antigen-recognition function, which is carried out by one end of the molecule that binds to antigen, i.e., the two Fab’s
- (2) and an effector function, which is performed by the other end of the molecule by interaction with phagocytic cells, other effector cells and mediator molecules (for example, complement), i.e., the Fc end
- Both these functions are extremely important during the immune response
- The antigen-recognition property of the immunoglobulin molecule confers its exquisite specificity to react with different molecular structures: epitopes which are either linear or more often of a conformational configuration, i.e., antigen
- For example, antibodies directed against the influenza virus recognize neuraminidase (N) and hemagglutinin (H), viral components whose neutralization prevents the virus from adhering to respiratory epithelial cells, thereby destroying the infectivity of the virus.
- This is achieved by producing two separate sets of antibodies, one directed at the N and the other at the H, with different amino acid sequences, each recognizing one of the two unrelated target antigens.
- Antigen recognition by the B cell occurs both at its cell surface through the BCR and by its secreted immunoglobulin product; in contrast, antigen recognition by the T cell occurs only through its surface receptor, i.e., the TCR
- During the course of an immune response to an immunogen specific classes of antibodies are generated at temporally different time periods
- For example, IgM antibody is synthesized early and IgG later by a process referred to as immunoglobulin class switching or isotype switching.
- The genetic mechanism by which this class switching occurs is referred to as somatic recombination or V(D)J recombination
- The effector function of an immunoglobulin is related to the specific isotype produced.
- For example, IgM antibodies synthesized early in the immune response because of their large molecular size are found and function best within the vascular system.
- Other immunoglobulins of lower molecular weight, e.g., the IgG antibodies, produced later in the immune response can readily diffuse between the intravascular and interstitial tissues where they function most effectively.
- Still other antibody molecules, i.e., the secretory IgA, are found at mucosal surfaces where they are produced and function as first lines of defense against pathogens that enter through the mucosal route, as exemplified by influenza, rhinoviruses, and HIV.
The Family of Immunoglobulins Includes Five Functional Classes of Antibodies
- The five different isotypes constitute a family of immunoglobulins, each with a different structure and a different function.
- The individual classes also referred to as isotypes are designated IgG, IgA, IgM, IgD, and IgE.
IgM Is Produced Rapidly Early in the Humoral Response
- IgM is the largest of the immunoglobulin molecules, present in the serum as a pentamer with ten antigen-binding sites and, because of its large size, is restricted almost entirely to the intravascular space.
- These macromolecules are highly efficient agglutinators of particulate antigens, e.g., bacteria and red blood cells, and they activate complement through the classical pathway with a high degree of efficiency.
- This class of immunoglobulin appears to be of greatest importance early in the primary immune response.
- When a foreign antigen is introduced into a host for the first time, the rapid synthesis of IgM antibodies ensures protection before IgG are produced.
- The transition from the production of IgM to IgG and the other isotypes occurs through a ‘‘class switch mechanism’’ involving the interaction of a cascading set of hypermutational events
- The duration of IgM synthesis peaks within a few days and the level of specific serum IgM declines more rapidly than the level of IgG antibodies.
- In one of the primary immune deficiencies, hyperimmunoglobulinemia M syndrome (HIGM), the congenital absence of the CD40 ligand on T cells or of the CD40 molecule on B cells results in the failure of the switch from IgM to IgG, causing the hyperproduction of IgM antibody with diminished production of IgG and the other isotypes, resulting in susceptibility to recurrent bacterial infection
- See Immunopaedia Case Study: An 8 year old boy with recurrent respiratory infections
- A maturational delay in the development of the CD40 receptor in the immature fetus and infant is thought to account for the prominent IgM production characteristic of the fetal and newborn immune responses (see Chapter 2).
IgG Is the Predominant Serum Antibody
- The IgG are the most abundant of the immunoglobulins and achieve significant concentrations in both the vascular and extravascular spaces.
- They have a relatively long half-life (t1/2) of ~23 days, cross the placenta, and are able to activate complement through the classical pathway
- This class of immunoglobulin, through its antigen-recognition function, is thought to contribute to protective immunity against many infectious agents, including bacteria, viruses, parasites, and some fungi.
- In addition to its role in the blood, IgG also provides antibody activity in tissues by exerting its effector function.
- Table 1 shows the different IgG isotypes, the concentrations of each and their functional properties.
Table 1. Properties of Human IgG Subclasses
| Property | IgG1 | IgG2 | IgG3 | IgG4 |
|---|---|---|---|---|
| Normal serum concentration (mg/dL) | 540 | 210 | 58 | 60 |
| Serum half-life (t½ ) days | 21 | 20 | 7 | 21 |
| Fc binding capacity on phagocytes | + | - | + | +/- |
| Activation of classical complement pathway | ++ | + | +++ | - |
| Capacity to cross placenta | +++ | + | ++ | +/- |
| Antibody activity | Protein antigens (e.g., diphtheria and tetanus) | Polysaccharide antigens (e.g., pneumococcal and H. influenzae ) | Protein antigens (e.g., diphtheria and tetanus) | Polysaccharide antigens (e.g., pneumococcal and H. influenzae ) |
- In the human, receptors for the Fc region exist on several types of phagocytic cells, including monocytes, macrophages, dendritic cells, neutrophils, and some lymphocyte subsets, such as NK cells (i.e., lymphocytes that carry out ADCC) (Table 2).
- Target cells coated with IgG antibodies directed against cell surface antigens may be eliminated through this ADCC mechanism.
- This occurs through the action of cytotoxic NK cells, which bind to the surface-coated IgG antibodies through their Fc receptor, thus allowing these cells to come into close contact with and kill the target cells by apoptosis.
Table 2. Summary of the different classes of Fc receptors for each of the major immunoglobulin isotypes together with their molecular and biological properties.
| Receptor name | Principal antibody ligand | Affinity for ligand | Cell distribution | Effect(s) following binding to antibody |
|---|---|---|---|---|
| FcγRI (CD64) | IgG1 and IgG3 | High (Kd ~ 10 −9 M) | Macrophages | Phagocytosis |
| Neutrophils | Cell activation | |||
| Eosinophils | Activation of respiratory burst | |||
| Dendritic cells | Induction of microbe killing | |||
| FcγRIIA (CD32) | IgG | Low (Kd > 10 −7 M) | Macrophages | Phagocytosis |
| Neutrophils | Degranulation (eosinophils) | |||
| Eosinophils | ||||
| Platelets | ||||
| Langerhans cells | ||||
| FcγRIIB1 (CD32) | IgG | Low (Kd > 10 −7 M) | B Cells | No phagocytosis |
| Mast cells | Inhibition of cell activity | |||
| FcγRIIB2 (CD32) | IgG | Low (Kd > 10 −7 M) | Macrophages | Phagocytosis |
| Neutrophils | Inhibition of cell activity | |||
| Eosinophils | ||||
| FcγRIIIA (CD16a) | IgG | Low (Kd > 10 −6 M) | NK cells | Induction of antibody-dependent cell-mediated cytotoxicity (ADCC) |
| Macrophages (certain tissues) | Induction of cytokine release by macrophages | |||
| FcγRIIIB (CD16b) | IgG | Low (Kd > 10 −6 M) | Eosinophils | Induction of microbe killing |
| Macrophages | ||||
| Neutrophils | ||||
| Mast cells | ||||
| Follicular dendritic cells | ||||
| FcεRI | IgE | High (Kd ~ 10 −10 M) | Mast cells | Degranulation |
| (high affinity) | Eosinophils | |||
| Basophils | ||||
| Langerhans cells | ||||
| Smooth muscle | ||||
| FcεRII (CD23) | IgE | Low (Kd > 10 −7 M) | B cells | Functions as a possible adhesion molecule |
| (low affinity) | T cells (on activated cells) | |||
| Eosinophils | ||||
| Langerhans cells | ||||
| Epithelial cells | ||||
| FcαRI (CD89) | IgA | Low (Kd > 10 −6 M) | Monocytes | Phagocytosis |
| Macrophages | Induction of microbe killing | |||
| Neutrophils | ||||
| Eosinophils | ||||
| Fcα/μR | IgA and IgM | High for IgM, Mid for IgA | B cells | Endocytosis |
| Mesangial cells | Induction of microbe killing | |||
| Macrophages | ||||
| FcRn | IgG | Monocytes | Transfers IgG from a mother to fetus through the placenta | |
| Macrophages | Transfers IgG from a mother to infant in milk | |||
| Dendritic cells | Protects IgG from degradation | |||
| Epithelial cells | ||||
| Endothelial cells | ||||
| Hepatocytes |
Antigen-Independent B Cell Development
- Lifespan of a B cell may be divided in an antigen-independent and an antigen-dependent phase.
- In the antigen-independent phase, a hematopoietic stem cell undergoes a series of divisions, giving rise to the generation of a large and diverse repertoire of daughter clones of B cells of increasing maturity, i.e., pro-B cell, pre-B cell, and immature B cell, characterized by the acquisition of certain surface markers, e.g., CD19 and BCR.
- The genes responsible for immunoglobulin synthesis are rearranged during the antigen-independent phase . This process continues during the antigen-dependent phase, when antigen is encountered and during which immunoglobulin genes undergo additional genetic modifications (somatic mutation and isotype switching).
- During this antigen-independent phase, the genes encoding for the heavy and light chains (V, D, and J) are cut and joined by DNA recombination, known as somatic recombination (Figure 2).
- The recombination of V, D, and J gene segments is carried out by a process called variable (diversity) joining [V(D)J] recombination and is directed by a set of enzymes called recombination activating gene-1, recombination activating gene-2 (RAG-1 and RAG-2)
- RAG-1 and RAG-2 recognize sequences called recombination signal sequences (RSSs) that flank the 3′ side of V segment, both sides of the D segment, and the 5′ side of the J segment.
- A successful heavy-chain gene rearrangement resulting in the synthesis of a functional pre-BCR stops VH to DJH rearrangement, and the pre-B cell begins to divide into a clone of cells, all expressing the same H chain.
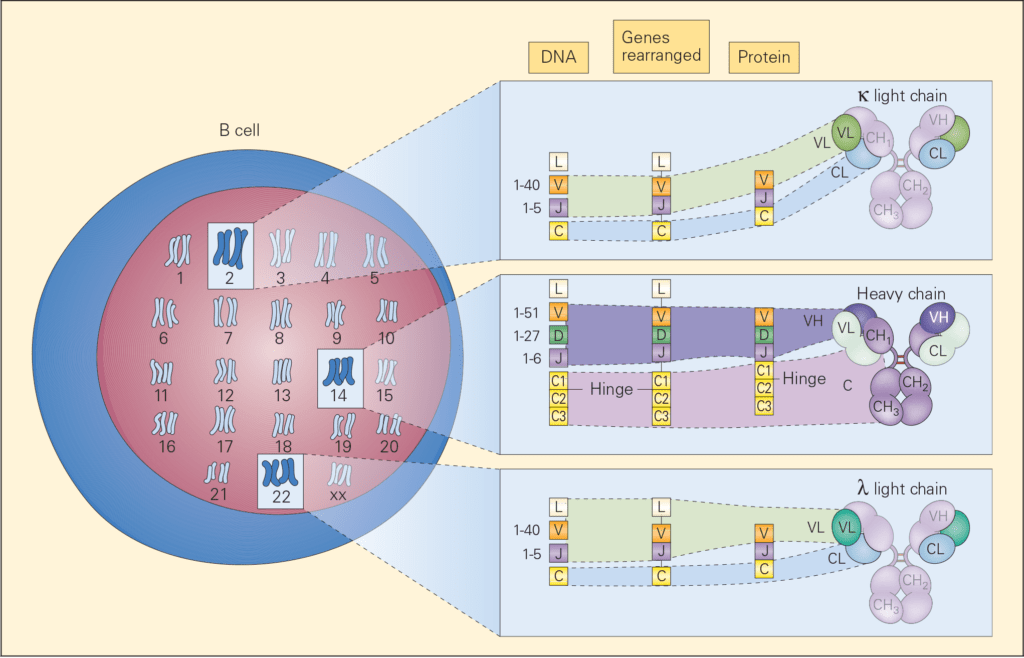
Figure 2. Schematic representation of the chromosomal locations of genetic loci, which control immunoglobulin synthesis together with the progressive steps of gene rearrangement, transcription, and translation involved in the synthesis and assembly of the various parts of an immunoglobulin molecule. The loci for light chains are found on chromosome 2 (kappa) and chromosome 22 (lambda) and for the heavy chain on chromosome 14. [Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].
- Recombinases are again re-expressed and the cells randomly join V and J gene segments on the kappa chain locus. After LC expression with the H chain signals the B cell to stop expressing recombinase; at this stage it is called an immature B cell and has expressed IgM on its membrane.
- The random events occurring during rearrangement and assembling of the BCR may, some times, lead to the production of autoreactive B cells responsible for autoimmune disease.
- Elimination of these potentially damaging specificities (negative selection) is ensured by two mechanisms known as clonal deletion and receptor editing.
Antigen-Dependent B Cell Development
- As the B cells enter the lymph nodes via the T cell areas, they encounter antigen, which binds to their BCR and stimulates the B cell to begin proliferating.
- Some B cells at this stage become short-lived IgM secreting plasma cells. Most B cells endocytose their antigen-BCR complexes, process the antigen, and present it on their membranes in combination with MHC-II.
- Protein antigens require the participation of Th2 cells, whereas nonprotein antigens activate B lymphocytes without the contribution of CD4+.
Somatic Hypermutation Results in B Cells with Higher Affinity Antibodies for Antigen, Resulting in Affinity Maturation
- Several elements contribute to the variability of the repertoire, including the large number of V, D, and J genes, their random rearrangement, the additional sequences created by the DNA repair mechanisms, and finally the combination of HC and LC.
- During the immune response, few B cells reacting to the antigen will start to proliferate in the germinal centers. At this time, the introduction of random mutations into the variable region of Ig genes (somatic hypermutation, SHM) results in the generation of B cells carrying a new BCR, which may have a lower or higher affinity for the antigen or have acquired self-reactivity.
- The interaction with the antigen on the surface of follicular dendritic cells, selects the cells with the highest affinity, useful to the ongoing immune reaction and which will generate plasma cells producing high affinity antibodies first of IgM and later of switched isotypes. This process is known as affinity maturation.
- Memory B cells carrying mutated and selected Ig genes will remain in the organism for a long time to prevent reinfection with the same pathogen.
Isotope Class Switch Recombination is Driven by T Cell Cytokines and Antigen
- Isotype class switching, begins with the binding of antigen to the surface IgM receptor, followed by signaling through a cascading set of transcription factors which then activate the expression of gene transcripts through DNA interactions to begin the synthesis of H chains on chromosome 14 and L chains on chromosomes 2 and 22 and final assembly and release of the component parts of the molecule into a complete immunoglobulin.
- When antigen interacts with a B cell without Th2 cell help, a continued synthesis of IgM alone occurs with no isotype switching, in contrast to that which occurs when B2 cells receive signals through Th2 help, where the subsequent IgG, IgA IgE progression is seen.
B Cell Activation and Antibody Production and the Antigen Antibody Reaction
- Immediately after the initial introduction of the immunogen, little or no antibody is detected in the serum. This period is referred to as the inductive or latent period.
- The processed antigen is presented to appropriate T cells for interaction with T or B cells for subsequent cell-mediated or humoral antibody production.
- The earliest primary response to most immunogens is characterized by the predominance of IgM antibody; the IgG class of antibody appears somewhat later and is followed even later still by the production of IgA and IgE. IgM antibody production is usually transient, and within two weeks after the initiation of the immune response, IgG antibody predominates.
- The IgM antibody formed early during the immune response has a low affinity, in contrast to the IgG antibody of the late immune response, which has increased affinity and avidity.
- Upon a subsequent exposure of a previously immunized host to the same immunogen, weeks, months, or even years later, there is a markedly enhanced response that is characterized by the accelerated appearance of immunocompetent T and B cells referred to as “memory” cells.
- Although IgM is produced initially in both the primary and secondary immune responses, the duration and magnitude of IgM production in the secondary immune response is transient and is associated with a greatly enhanced production of antibody that is primarily of the IgG isotype.
- Subsequent antibody production is then converted from IgG to other immunoglobulin isotypes by isotype switch.
- In the secondary immune response, the memory B cells that have already switched their Ig isotype immediately begin to secrete that isotype.
Quiz
Related Talk
Guido Ferrari, Duke University – ADCC and Antibody functions