In a recent study, researchers have described promising new approach for predicting whether an individual will develop symptoms after contracting the influenza virus (Figure 1). Unlike current methods, which primarily rely on assessing antibody levels, this novel technique focuses on immune cells present in a person’s body several months before flu infection occurs.
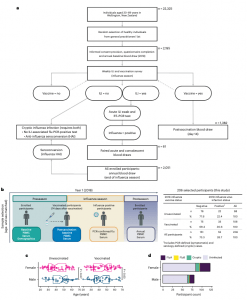
Figure 1: SHIVERS-II study design, participant enrollment, sample collection and participant demographics. a, Schematic depiction of the SHIVERS-II study design and participant numbers for year 1 (2018). RT–PCR, reverse transcription followed by PCR. b, Following consented enrollment, demographic information and whole blood samples were collected from all vaccinated and unvaccinated participants in the preseason period (nonvaccinated baseline) and 14 days after vaccination (vaccinated baseline). Participants meeting the WHO-defined criteria for ILI were tested for influenza viruses by PCR, and confirmed cases were sampled further during acute infection. All enrolled participants were sampled after the season. Cryptic infections were adjudicated in the postseason period from ILI- and PCR-negative participants with a fourfold or greater increase in HAI antibody titers without postvaccination HAI seroconversion. Right, 206 enrolled participants were selected for study inclusion from four baseline comparator groups (unvaccinated–uninfected, unvaccinated–infected, vaccinated–uninfected and vaccinated–infected) based on age- and sex-matching. c, Sex (assigned at birth) of n = 206 participants stratified by vaccination status and age (years) and compared by two-sided Wilcoxon rank-sum test (unvaccinated female (n = 58) versus unvaccinated male (n = 42), P = 0.07; vaccinated female (n = 66) versus vaccinated male (n = 42), P = 0.63). Boxes represent the median and 25th–75th percentiles; whiskers indicate the minimum (left) and maximum (right) values no further than 1.5 times the interquartile range (IQR); notches extend to 1.58 × IQR/sqrt(n), providing the 95% confidence interval (CI). P < 0.05 indicates significance. d, Participants’ sex stratified by influenza virus infection status and strain. Influenza A (FluA) viruses include A(H1N1) and A(H3N2) strains; influenza B (FluB) viruses include the B/Victoria lineage and B/Yamagata lineage strains.
The study’s key discovery was that specific immune cells could more accurately forecast an individual’s susceptibility to flu symptoms. Interestingly, the presence of a functionally diverse set of immune cells was linked to enhanced protection against flu symptoms. To identify these cells, the researchers compared immune cell profiles in the blood of individuals who developed flu symptoms to those who remained asymptomatic or uninfected.
This study included participants from the Surveillance for Community Cohort-Based Influenza-Like Illness (SHIVERS-II) study in New Zealand, further reinforcing its findings. Moreover, the research highlighted the effectiveness of flu vaccination in bolstering protective anti-flu immune cells, thereby reducing the likelihood of symptom development. Intriguingly, unvaccinated individuals who managed to avoid symptoms displayed immune cell profiles resembling those of vaccinated individuals with strong protection. This insight underscores the importance of vaccination as the most reliable means of symptom prevention and offers a valuable tool for encouraging vaccine uptake by accurately assessing individual susceptibility.
In essence, this study reaffirms the critical role of vaccination in mitigating influenza symptoms and underscores the significance of specific immune cell levels in correlating with this protection.
Journal article: Mettelman, R.C., 2023. Baseline innate and T cell populations are correlates of protection against symptomatic influenza virus infection independent of serology. Nature Immunology.
Summary by Stefan Botha










