 The SARS-CoV-2 501Y.V2 (B.1.135) variant emerged in South Africa from approximately October 2020 (Tegally et al., 2020) and has since become the dominant variant in the country. This variant contains a number of mutations within the antibody epitopes of key neutralizing antibodies including N501Y, E484K and K417N mutations within the receptor-binding domain (RBD), an immunodominant region of the SARS-CoV-2 spike. The 501Y.V2 variant also carries key mutations in the N-terminal domain (NTD) – another region of the spike protein that is a target of neutralizing antibodies.
The SARS-CoV-2 501Y.V2 (B.1.135) variant emerged in South Africa from approximately October 2020 (Tegally et al., 2020) and has since become the dominant variant in the country. This variant contains a number of mutations within the antibody epitopes of key neutralizing antibodies including N501Y, E484K and K417N mutations within the receptor-binding domain (RBD), an immunodominant region of the SARS-CoV-2 spike. The 501Y.V2 variant also carries key mutations in the N-terminal domain (NTD) – another region of the spike protein that is a target of neutralizing antibodies.
Neutralizing antibodies are likely the correlate of protection in SARS-CoV-2 vaccines and therefore understanding the effect of this variant on antibody responses has major implications for vaccine rollout and immunogen design.
Wibmer et al. (2021) studied the effect of the 501Y.V2 lineage on the neutralization potency of monoclonal antibodies and plasma samples from individuals infected with the unmutated virus (Wibmer et al., 2021). They showed that the 501Y.V2 variant escaped the neutralizing antibody responses of antibodies directed both to the RBD and NTD regions. Many other studies have illustrated that reduced neutralisation capacity of the 501Y.V2 variants are partly attributed to the E484K and K417N mutations. These data suggest the possibility that the current SARS-CoV-2 vaccines that are based on the unmutated virus could have reduced efficacy in protecting against infection by the 501Y.V2 variant. The study by Wibmer et al. (2021) did show the presence of binding antibodies still active in the serum which further suggests that other antibody functions may still be able to recognize this new variant. Such functions in theory may not be able to block entry of the virus but could help in reducing disease severity.
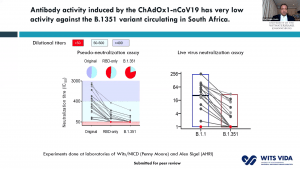
Madhi et al. (2021) went on to show that the ChAdOx1-nCoV19 vaccine – made by the University of Oxford and Astra Zeneca – was ineffective in protecting against mild/moderate COVID-19 disease (Madhi et al., 2021). Plasma samples from individuals vaccinated with two doses of the vaccine had reduced neutralization potency towards the 501Y.V2 variant; with neutralization potency being abrogated completely in some samples. The effect of the vaccine on severe COVID-19 disease is still under investigation.
These studies suggest that current vaccines may have a lower efficacy against emerging variants e.g. SARS-CoV-2 501Y.V2 (B.1.135) that contain mutations such as E484K and K417N, or any other mutation that reduces the neutralization capacity of vaccine-induced or convalescent antibodies. More research is required to determine if current vaccines have efficacy against severe COVID-19 (disease state associated with high mortality). If they do, this provides evidence for rolling out vaccines to protect against severe COVID-19.
Also read:
- Do mutations in SARS-CoV-2 variants reduce the functional activity of mRNA-vaccine elicited Abs?
- Are current putative COVID-19 vaccines effective against the B.1.351 variants?
- Mutations in SARS-Cov-2 B.1.351 variant reduces vaccine induced Ab neutralisation
- Not all SARS-CoV-2 mutations lead to reduced antibody neutralisation capacity
References
- Madhi et al., 2021. Safety and efficacy of the ChAdOx1 nCoV-19 (AZD1222) Covid-19 vaccine against the B. 1.351 variant in South Africa. medRxiv.
- Tegally et al., 2020. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage w
- Wibmer et al., 2021. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. BioRxiv.
Summary by Thandeka Moyo-Gwete










