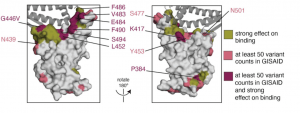
Caption: Surface representations of the RBD (PDB 6M0J). Sites where mutations have a strong effect on binding, have circulating variation with >50 total counts in GISAID, or both, are colored in olive, pink, or maroon, respectively. See Methods for precise description of highlighted sites. ACE2 is shown as a dark gray cartoon. (Source: Greaney et al., Pre-print)
The emergence of rapidly spreading SARS-CoV-2 variants in many countries is causing public concern. These variants, such as B.1.351 and B1.1.7, first detected in South Africa and the United Kingdom respectively , contain multiple mutations in various sites of the SARS-CoV-2 genome including in the receptor-binding domain of the spike protein. Researchers are currently studying the implication of these variants on SARS-CoV-2 transmission, pathogenesis and immunity. Reports (pre-prints) suggest that variants that contain the N501Y mutation such as B.1.1.7 and S.501Y.V2 are associated with increased binding affinity to ACE-2 (human cell target, Cheng et al., 2021) and are potentially more transmissible (spread faster) than other SARS-CoV-2 variants. An additional concern is whether vaccines developed based on the initial circulating SARS-CoV-2 variant would also confer immunity against emerging variants. In this summary, we provide evidence from that not all mutations lead to immune escape.
Study by Greaney et al., aimed to determine how convalescent serum antibodies are impacted by all mutations to the spike’s receptor-binding domain (RBD), the main target of serum neutralizing activity. They showed that mutations that cause a reduction in antibody binding affinity typically occur in RBD region, however majority do not lead to a reduction in antibody neutralisation capacity. They demonstrated that the E484 mutation, which is also present in the 501.V2 variant and another emerging variant in Brazil, results in a 10-fold reduction in neutralisation capacity of convalescent sera from some patients but not others. However, they observed no negative antibody binding effect of the N501Y mutation, a finding also observed by others (Xie et al., 2021). Specifically, Xie et al., showed that the neutralisation capacity of BNT162b2 (Pfizer/BioNTech) vaccine-induced antibody immunity from 20 participants had equivalent neutralizing titers to the N501 and Y501 viruses. Thus providing hope for that current vaccine based on the initial SARS-CoV-2 circulating variant, could protect against emerging variants.
In summary, these studies suggest that some mutations do result in a reduction in immune recognition and neutralisation. However, this represents a potential immune escape from a single epitope. The combined anti-SARS-CoV-2 immunity conferred by other antibodies not specific to epitopes that contain E484 mutation may still confer favourable immunity.
Articles:
- Greaney et al., Pre-Print. Comprehensive mapping of mutations to the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human serum antibodies. bioRxiv
- Xie et al., Pre-Print. Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera. bioRxiv
Summary by Cheleka Mpande










