Systemic lupus erythematosus (SLE), the most prevalent type of the chronic autoimmune illness lupus, may be treated using a novel strategy that targets iron metabolism in immune system cells (Figure 1). In a mouse model of SLE, researchers have now learned that inhibiting an iron absorption receptor lowers disease pathology and boosts the activity of regulatory T cells that fight inflammation.
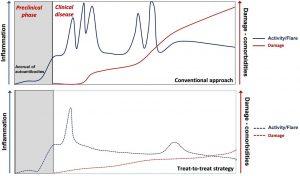
Figure 1: Natural history of SLE and the potential impact of a treat-to-target strategy. The disease starts with a preclinical, asymptomatic phase characterised initially by the appearance of autoantibodies common to all autoimmune diseases and, later, of lupus-specific autoantibodies. Subsequent clinical course is characterised by periods of variable disease activity (measured by SLE disease activity indices), with frequent flares resulting in inflammation-driven irreversible damage. Damage—measured by the SLICC/ACR damage index—increases the morbidity and mortality in SLE. Damage is driven initially by inflammation and later—with progression of the disease—also by therapy. With time, comorbidities such as infections, premature atherosclerosis and malignancies become an important part of the disease burden. Effective therapy targeting low-disease activity or remission has the potential to decrease the frequency and severity of lupus flares and resulting damage. ACR, American College of Rheumatology; SLE, systemic lupus erythematosus (Source: Boumpas, D.T., et al., 2021).
When an individual’s own healthy tissues are attacked by their immune system, lupus, including SLE, results, resulting in discomfort, inflammation, and tissue destruction. Skin, joints, brain, lungs, kidneys, and blood vessels are the most typical organs that lupus damages. Lupus treatments focus on symptom management, lowering immune system tissue assault, and organ damage prevention. Belimumab, a single targeted biologic drug, was licenced in 2011 to treat SLE.
The CRISPR genome editing screen was used by the researchers to assess the iron-handling genes in T cells. They determined that inflammatory T cells require the transferrin receptor, which imports iron into cells, but regulatory T cells that fight inflammation do not. The transferrin receptor was shown to be more strongly expressed on T cells from SLE-prone mice and T cells from SLE patients, which led to the cells’ excessive iron accumulating.
A transferrin receptor-blocking antibody decreased intracellular iron levels, reduced pro-inflammatory T cell activity, and increased regulatory T cell activity. Mice predisposed to SLE were treated with the antibody, which decreased liver and kidney disease and raised IL-10 production.
Journal article: Voss, K., et al., 2023. Elevated transferrin receptor impairs T cell metabolism and function in systemic lupus erythematosus. Science Immunology.
Summary by Stefan Botha










