In a new study, researchers have made a new discovery regarding Aedes aegypti mosquitoes, shedding light on a potential breakthrough in the battle against mosquito-borne viruses like dengue, yellow fever, and Zika (Figure 1). These viruses, often devastating and sometimes fatal for humans, are spread via Ae. aegypti mosquitoes, which remarkably remain unaffected by these pathogens. The research centers on a crucial Ae. aegypti protein, Argonaute 2, which plays a pivotal role in bolstering the mosquitoes’ resilience and continued mobility in the face of viral infections.
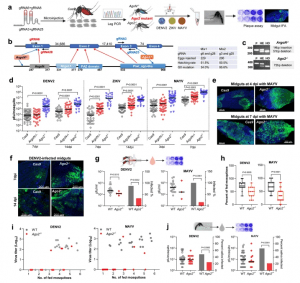
Figure 1: Generation of Ago2 knockout Ae. aegypti lines and the effect of Ago2 disruption on arbovirus infection and transmission. a flowchart of experiment design. b gene structure of Ago2, predicted functional domains, and guide RNA (gRNA) design for CRISPR/Cas9. The table shows data from generating Ago2 knockout lines. c Ago2 amplification in Ago2 knockout lines. P, parental line Cas9; HE, heterozygous mutants; HO, homozygous mutants. d virus titer in Ago2 knockout (Ago2−/− and ArgoN−/−) and Cas9 mosquitoes on various days post-infection (dpi) with DENV2, ZIKV, or MAYV. IFA detection of MAYV (e) and DENV2 (f) antigen in midguts of Cas9 and Ago2−/− mosquitoes at different days post virus infection. MAYV and DENV2 were detected with the corresponding monoclonal antibody (green). Nuclei were stained with DAPI (blue). g virus titer and infection prevalence in the feeding solution collected from a feeder exposed to a group of females at 14 dpi with DENV2 or 5 dpi with MAYV. The Liverpool strain was used as a control (WT). For DENV2 infection, n = 16 for WT and n = 15 for Ago2−/−; for MAYV infection, n = 19 for both WT and Ago2−/−. h percentage of the fed mosquitoes in each group of females at 14 dpi with DENV2 or 5 dpi with MAYV. Data were presented as box and whiskers (Min to Max). i virus titer plotted against the number of the fed mosquitoes in each cup. j virus titer and infection prevalence of individual saliva samples collected from Ago2−/− and WT at 14 dpi with DENV2 or 5 dpi with MAYV. For DENV2 infection, n = 62 for WT and n = 61 for Ago2−/−; for MAYV infection, n = 56 for both WT and Ago2−/−. Each experiment comprised at least two biological replicates, and the data were pooled for generating the graphs. Virus titers were determined by plaque assay, and horizontal lines indicate the medians of the virus titer (d, g and j). P values were determined by an unpaired two-sided Mann-Whitney test for virus titer, an unpaired two-sided Fisher’s exact test for infection prevalence, or an unpaired two-sided t-test for (h). Source data are provided as a Source Data file.
The findings represent a significant leap forward in our comprehension of mosquito biology. Moreover, they hint at a novel strategy – dismantling Ae. aegypti mosquitoes’ defenses upon viral infection. By rendering these mosquitoes less impervious to viruses, their ability to transmit diseases could be severely impaired, potentially curbing the virus’s spread to humans. This approach is distinct from the conventional practice of bolstering mosquito resistance to viruses.
Ae. aegypti mosquitoes are notorious for transmitting arboviruses, including dengue, yellow fever, Zika, chikungunya, and Mayaro viruses. Each year, these pathogens afflict millions of individuals globally, with tens of thousands succumbing to the diseases they cause. Currently, there are no antiviral therapies available for these viruses, except for a vaccine against yellow fever and a dengue vaccine for specific populations. Control methods primarily rely on insecticides, which have encountered limited success and led to the emergence of insecticide-resistant mosquitoes.
The research zeroes in on Argonaute 2 (Ago2), a critical protein that operates within Ae. aegypti mosquitoes as part of the siRNA pathway, a potent antiviral mechanism that identifies and destroys viral RNAs. In mosquitoes where the Ago2 gene is absent, the siRNA pathway is inhibited, rendering arbovirus infections more severe. Mosquitoes with compromised siRNA pathways become less capable of transmitting viruses, as they fall ill, reduce their feeding activity, and often perish within days.
This breakthrough research holds promise for advancing our strategies against mosquito-borne viruses. By targeting the vulnerabilities in Ae. aegypti mosquitoes, we may finally gain the upper hand in mitigating the transmission of these devastating diseases. Instead of focusing on making mosquitoes more virus-resistant, we may be on the brink of rendering them more susceptible, offering a glimmer of hope in our quest to thwart the spread of mosquito-borne viruses.
Journal article:Dong, S., et al. 2023. Aedes aegypti Argonaute 2 controls arbovirus infection and host mortality. Nature Communications.
Summary by Stefan Botha










