Systemic sclerosis, a rare autoimmune disease, is known for its clinical complexity—patients can present with symptoms ranging from mild skin thickening to life-threatening damage to internal organs like the lungs and kidneys. Now, researchers in Japan have uncovered a potential explanation for this variability: distinct immune cell signatures may underlie the differences in disease severity.
Systemic sclerosis is characterized by immune-mediated fibrosis (tissue scarring), blood vessel damage, and skin changes such as thickening and Raynaud’s phenomenon. However, in some patients, the disease extends beyond the skin to damage vital organs—complications that can significantly worsen prognosis.
In a new study, a team used single-cell analysis to identify specific immune cell populations that correlate with different systemic sclerosis outcomes (Figure 1). Their findings highlight how immune dysregulation at the cellular level may drive complications in some patients while sparing others.
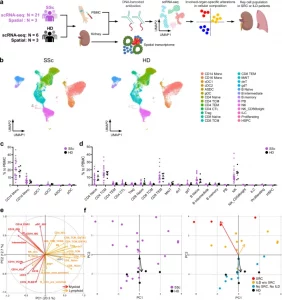
Figure 1: Comprehensive single-cell analysis reveals distinct immune profiles in patients with systemic sclerosis and healthy donors. a Overview of the experimental workflow. Created in BioRender. Shimagami, H. (2025) https://BioRender.com/jhwa38f. SSc systemic sclerosis, HD healthy donor, PBMC peripheral blood mononuclear cells, scRNA-seq single-cell RNA sequencing, SRC scleroderma renal crisis, ILD interstitial lung disease. b UMAP plots displaying CITE-seq data of PBMCs from SSc patients (SSc; n = 21) and HDs (HD; n = 6), annotated with reference mapping. Each plot shows 30,000 randomly selected cells. Mono monocytes, cDC conventional dendritic cells, ASDC AXL+ dendric cells, pDC plasmacytoid dendritic cells, TCM central memory T cells, TEM effector memory T cells, CTL cytotoxic T lymphocytes, Treg regulatory T cells, MAIT mucosal associated invariant T cells, dnT double negative T cells, gdT gamma-delta T cells, PB plasmablasts, NK natural killer cells, ILC innate lymphoid cells, Proliferating proliferating cells, HSPC hematopoietic stem and progenitor cells. Percentages of myeloid (c) and lymphoid (d) cell populations relative to the total PBMCs in SSc patients (n = 21, purple) and HDs (n = 6, black). Values represent means with standard error of the mean (SEM). Statistical analysis was conducted using the two-sided Kolmogorov–Smirnov test with Bonferroni correction for multiple comparisons. e Principal component analysis on the proportion of each cell subset to the total PBMCs. Each subset was defined based on CITE-seq data (Supplementary Figs. 3–8). Vectors represent Principal Component (PC) 1 and PC2 for each cell subset. Red, myeloid cell subset; orange, lymphoid cell subset; gray, HSPC. Vectors with lengths greater than 0.3 are displayed. f Distribution of values of PC1 and PC2 for each study participant. In the left panel, dots represent individuals, with SSc patients shown in purple and HDs in black. In the right panel, each SSc patient is indicated by a colored dot: red for SRC patients, orange for ILD patients without SRC (ILD w/o SRC), and blue for patients without both SRC and ILD (No SRC, No ILD); HDs are represented by black dots. Arrows show the mean vectors for each group, colored to match the dots of corresponding group. Source data are provided as a Source Data file.
To address this, the researchers analysed blood and tissue samples from affected individuals using single-cell RNA sequencing and surface protein profiling. This approach enabled them to dissect immune cell populations with high resolution and uncover gene expression patterns linked to clinical outcomes.
Their investigation revealed two immune cell subtypes strongly associated with specific organ complications:
- EGR1-expressing CD14+ monocytes were enriched in patients with scleroderma renal crisis, a severe kidney manifestation. These monocytes appear to differentiate into pro-inflammatory macrophages that promote fibrosis and inflammation around the kidneys.
- CD8+ T cells with a type II interferon signature were linked to progressive interstitial lung disease. This subset exhibited heightened inflammatory potential and is likely involved in the recruitment of additional immune factors that damage lung tissue.
By linking specific immune cell profiles to disease manifestations, the study lays the groundwork for a more personalized approach to managing systemic sclerosis. The presence of biomarkers such as EGR1-expressing monocytes or interferon-signature T cells could help clinicians predict which patients are at greater risk for organ involvement—potentially enabling earlier intervention.
Few effective treatments currently exist for systemic sclerosis, and therapies that prevent organ fibrosis remain a major unmet need. This research opens the door to new strategies that could target the specific immune mechanisms driving disease progression in individual patients.
Journal article: Shimagami, H. et al. 2025. Single-cell analysis reveals immune cell abnormalities underlying the clinical heterogeneity of patients with systemic sclerosis, Nature Communications.
Summary by Stefan Botha










