The gut’s immune system is constantly fine-tuned throughout life, with immunoglobulin A (IgA), the primary antibody at mucosal surfaces, undergoing continual diversification. Traditionally, this process has been attributed mainly to microbial stimulation, but new research reveals that diet alone can drive long-lasting changes in IgA responses, independent of the gut microbiota (Figure 1).
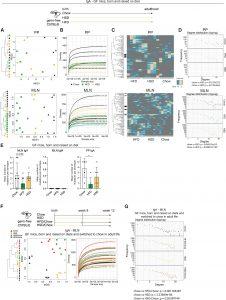
Figure 1 Purified diets induce a decrease in IgA repertoire diversity within GCs in intestinal lymphoid organs of GF mice. Adult C57BL/6J GF offspring of dams fed either chow or the purified diets during pregnancy and lactation. Upon weaning, the offspring continued to be fed the same diet as their mothers until analysis at 10 weeks of age. Immunoglobulin repertoire sequencing for IgA was performed on MLNs and Peyer’s patches (PPs).
(A) Hierarchical clustering trees of CDR3 amino acid sequence similarities (left), multidimensional scaling (MDS) plots (right). (B) Rarefaction plots of immunoglobulin diversity. (C) Top 100 shared IgA clonotypes based on immunoglobulin heavy-chain CDR3 amino acid sequences. Clonotypes falling into high-degree clusters are indicated with a black bar on the left side (degree cutoff 10 for PP, 30 for MLN, in accordance with their respective clonotype diversity as seen in the rarefaction plots). Clonotype sequences of the heatmap are listed in Table S2. (D) Degree distribution of shared CDR3 amino acid clonotypes in PP and MLN. The degree is the number of connections in a network of clonotypes connected by a Levenshtein distance of 1. (E) Mean number of mutations per clonotype for IgA in MLN and PP and for IgM in the MLN. (F) Adult GF mice, born and raised under purified diets, were switched to chow for 4 weeks at 8 weeks of age. Hierarchical clustering trees of CDR3 amino acid sequence similarities (left), multidimensional scaling (MDS) plots (middle), and rarefaction plots of immunoglobulin diversity (right) based on immunoglobulin heavy-chain CDR3 amino acid sequences for IgA in MLN are shown. (G) Degree distribution of shared CDR3 amino acid clonotypes for IgA in MLN. Mean indicated by bar height with standard deviation. p values (displayed if <0.05) determined by one-way ANOVA followed by Tukey’s multiple comparisons test. ∗p < 0.05. Each dot represents a single animal. Sample number n ≥ 3. Data are representative of two independent experiments.
Key Findings
- Diet shapes intestinal germinal center reactions even without microbial exposure
- Lipopolysaccharides (LPS) in food trigger TLR4 signaling, boosting mucosal IgA production and diversification
- LPS needs to be packaged in colloidal liposomes (not in free solution) to effectively drive this immune response
- Early-life exposure to dietary LPS imprints a lasting IgA repertoire
IgA antibodies in the gut are known for their high mutational load, a sign of their ongoing adaptation to new antigens. But researchers using germ-free mice found that even in the absence of microbial colonization, early-life diet composition alone could significantly shape IgA induction and diversification.
When mice were fed different types of diets, some based on grain-derived formulations and others made from purified macronutrients, the researchers discovered that trace lipopolysaccharide (LPS) contamination in certain diets was the key driver of intestinal germinal centre activation.
Dietary LPS, a bacterial cell wall component often presents as a contaminant in food processing, triggered Toll-like receptor 4 (TLR4) signalling in gut immune cells. This signalling ramped up mucosal germinal centre activity, which is crucial for IgA class-switching and somatic hypermutation.
Interestingly, the physical form of LPS mattered. Only when embedded within colloidal liposomes, tiny fat-based particles similar to those found in certain food formulations, did dietary LPS effectively induce IgA diversification. In contrast, free, dispersed LPS failed to elicit the same response.
The study also showed that early-life exposure to dietary LPS had a durable imprinting effect on the IgA repertoire, influencing the diversity and specificity of gut antibodies later in life.
This suggests that the formulation and composition of infant and early childhood diets, even in the absence of live microbes, can have lasting consequences for mucosal immune programming.
This research challenges the long-held view that microbial colonisation is the sole driver of mucosal immune diversification. It suggests that:
- Dietary components, especially those containing or contaminated with bacterial molecules like LPS, can prime gut immunity in early life.
- The physical structure of food formulations (e.g., colloidal liposomes) plays a critical role in how the immune system perceives dietary antigens.
- Early-life nutrition may shape not just metabolism but also long-term immune resilience, potentially influencing susceptibility to infections and immune disorders.
In the future, these insights could inform early-life dietary interventions, perhaps even designing foods that optimize gut immune development in newborns and infants, especially in settings where microbiota exposure is altered.
Journal article: Mooser, C., et al., 2025. Diet-derived LPS determines intestinal IgA induction and repertoire characteristics independently of the microbiota. Immunity.
Summary by Stefan Botha










