Glioblastoma, an aggressive brain tumour with a bleak prognosis, is currently incurable. Traditional therapies like chemotherapy and immunotherapy show limited effectiveness against this devastating cancer. However, researchers are developing a novel immunotherapy approach that offers a glimmer of hope for glioblastoma patients (Figure 1).
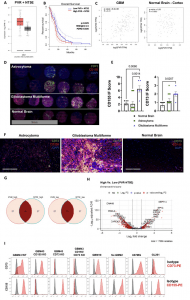
Figure 1: CD155 and CD73 are highly upregulated in GBM and represent negative prognostic factors. A Transcriptional gene expression TCGA data depicting co-expression levels of PVR and NT5E in GBM versus normal tissue; Tumor—n = 163 patients (min: 1.9, max: 5.7, median: 4.1, lower whisker bound: 2.1, upper whisker bound: 5.7, Q1: 3.6, Q3: 4.6), Normal—n = 207 patients (min: 1.1, max: 4.0, median: 3.1, lower whisker bound: 2.1, upper whisker bound: 4.0, Q1: 2.8, Q3: 3.4). B Kaplan-Meier survival plot of PVR/NT5Ehigh/high versus PVR/NT5Elow/low GBM patients (n = 81 patients/group; Mantel-Cox test). C Two-tailed Pearson correlation of PVR and NT5E expression in GBM and normal brain tissue; Tumor—n = 163 patients, Normal—n = 115 patients.D IF tissue array staining for CD155 and CD73 expression in astrocytoma (n = 12 cores), GBM (n = 6 cores), and normal brain tissue (n = 6 cores). E IF scoring of staining in Fig. 1D (ordinary one-way ANOVA, Tukey’s multiple comparison test). F Representative IF tissue array staining of CD155 and CD73 expression from Fig. 1D at 200x magnification. G Venn diagram of no. of patients in PVR/NT5Ehigh/high and PVR/NT5Elow/low groups from TCGA-GBM patient transcriptional gene expression data. (n = 156 patients). H Volcano plot of differentially expressed genes in PVR/NT5Ehigh/high and PVR/NT5Elow/low groups. I Histograms depicting CD73 and CD155 expression on GBM cell lines measured via flow cytometry. Data are presented as mean values +/- SEM. Source data are provided as a Source Data file.
Glioblastoma is almost universally fatal, with a median survival time of only 14 months. Existing treatment strategies, including those that have proven successful against other cancers, often fail to make a significant impact on glioblastoma.
Researchers are pioneering a novel immunotherapy that utilizes genetically engineered immune cells. Traditional cell therapies typically rely on autologous cells, meaning they are extracted from the patient, modified, and then reintroduced. Unfortunately, these autologous cell therapies have shown minimal to no effect on glioblastoma.
This new approach leverages allogeneic immune cells, which are engineered from a source other than the patient. In this study, the researchers used induced pluripotent stem cells (iPSCs) to create natural killer (NK) cells, a type of immune cell. These NK cells were then genetically modified to enhance their effectiveness.
The research team tested their engineered cells in mice with human brain tumours. The results were highly encouraging. Direct injection of these cells led to complete eradication of the tumours in the mice. This preclinical study demonstrates the remarkable potential of this new approach.
One of the major hurdles in cell-based therapies has been the difficulty and inefficiency of expanding patient-derived cells. The use of iPSCs eliminates this hurdle by providing a readily available and expandable cell source. This approach significantly simplifies the manufacturing process, paving the way for broader patient access.
With these promising preclinical findings, the next step is to conduct clinical trials to assess the safety and efficacy of this engineered cell therapy in glioblastoma patients. This includes patients whose tumours were not completely removed by surgery.
The goal of this research is to provide patients with glioblastoma a more effective and potentially life-saving treatment option. The potential of this therapy is significant, and researchers are committed to bringing it to the clinic to benefit patients in need.
Journal article: Lupo, K.B., et al., 2024. synNotch-programmed iPSC-derived NK cells usurp TIGIT and CD73 activities for glioblastoma therapy. Nature Communications.
Summary by Stefan Botha










