A recent study has revealed a surprising new role of our eyes: acting as immunological defenses for our brain. A new study sheds light on this novel function and its potential for future therapies (Figure 1).
Researchers discovered that injecting vaccines directly into the eyes of mice effectively neutralized the herpes virus, a major culprit behind brain inflammation. Interestingly, the vaccine triggered an immune response through lymphatic vessels located along the optic nerve.
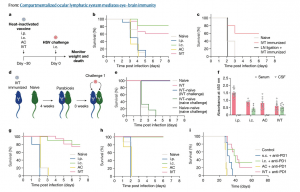
Figure 1: Antigens in the posterior eye elicit immune responses in the brain. a, Schematic of the schedule of procedures for the experiments described below. b, Wild-type C57BL/6J mice were immunized using heat-inactivated HSV-2 injection through i.p., i.c., AC and IVT administration. Survival was monitored after i.c. challenge with a lethal dose of HSV-2 30 days later (naive, n = 18; i.p., n = 12; i.c., n = 6; AC, n = 6; IVT, n = 18). c, dCLNs of mice were ligated using a cauterizer. Seven days later, mice were injected through the IVT route with heat-inactivated HSV-2. Their survival was monitored after i.c. challenge with a lethal dose of HSV-2 30 days later (naive, n = 5; IVT immunized, n = 5; LN ligation, n = 6). d, Schematic of the parabiosis mouse model and treatment plans. e, Mice were injected through the IVT route with heat-inactivated HSV-2. Four weeks later, the immunized mice were joined to naive mice. The immunized mice or naive mice were challenged through the i.c. route with a lethal dose of HSV-2 after 3 weeks, and their survival was monitored (naive, n = 4; IVT, n = 4; IVT–naive (IVT challenge), n = 6; IVT–naive (naive challenge), n = 2; naive–naive (naive challenge), n = 2). f, Anti-HSV-specific antibody was measured by enzyme-linked immunosorbent assay after different routes of HSV-2 immunization (i.p., n = 12; i.c., n = 6; AC, n = 10; IVT, n = 10). Data are shown as mean ± s.e.m. g, Wild-type C57BL/6J mice were injected with heat-inactivated HSV-1 through i.p., i.c., AC or IVT administration. Their survival was monitored after i.c. challenge with a lethal dose of HSV-1 30 days later (naive, n = 15; i.p., n = 6; i.c., n = 6; AC, n = 6; IVT, n = 18). h, As in a, but S. pneumoniae strain TIGR4 was used (naive, n = 8; i.p., n = 5; i.c., n = 5; AC, n = 8; IVT, n = 8). i, Mice were inoculated through the i.c. route with 50,000 GL261 luciferase-expressing (GL261–Luc) brain tumour cells, treated with irradiated GL261–Luc cells through s.c., i.c., AC or IVT administration (day 7) along with anti-PD1 (RMP1-14) antibodies (days 7, 9 and 11) and monitored for survival (naive, n = 6; s.c., n = 6; i.c., n = 6; AC, n = 6; IVT, n = 12). Data are representative of two independent experiments. The graphics in a,d were created with BioRender.com.
This finding described a previously unknown “shared immune response” between the brain and the eye. The study highlights the eyes’ potential advantage as a drug delivery route compared to the more challenging brain barrier.
The research team identified two distinct lymphatic systems within the eye, each regulating immune responses in different regions. When the mice received an inactivated herpes vaccine, the lymphatic vessels in the optic nerve sheath (at the back of the eye) sprang into action. This not only protected the mice from active herpes infections but also offered surprising defence against bacteria and even brain tumours.
The study’s key takeaway is the discovery of a shared lymphatic circuit enabling a unified immune response between the back of the eye and the brain. This under-explored aspect of the eye’s immune system opens exciting possibilities for developing novel therapeutic strategies, potentially tackling both eye and central nervous system diseases.
Journal article: Yin, X., et al., 2024. Compartmentalized ocular lymphatic system mediates eye–brain immunity. Nature.
Summary by Stefan Botha










