In a recent preprint, Warwicker investigated the degree to which the D614G mutation in the SARS-CoV-2 receptor-binding domain (RBD) allows access of spike (S) protein trimers to conformations/forms similar to the linoleic acid (LA)-bound locked conformations without LA binding.
Based on the analysis of mutations around the LA binding RBD pocket in the SARS-CoV-2 Omicron variant S protein is suggestive of certain changes that may occur in locked conformation accessibility without LA binding at a neutral pH (READ MORE: Inclusion of non-spike proteins in SARS-CoV-2 vaccines may be important for the induction of protective T cell memory, Do mutations in the SARS-CoV-2 spike protein enhances viral infectivity?).
Several studies have investigated the structure of the S trimer, understanding of how this structure is changed based on pH and the various mechanisms of this change in addition to how relates to its function remain to be elucidated.
In this study, they investigated a variety of S RBD structures of coronaviruses including those presenting with D614G mutations i.e. SARS-CoV-2 Delta and the Omicron variants. The researchers also closely inspected the LA binding RBD pocket to observe the open (RBD up) and closed (RBD down) conformations (Figure 1).
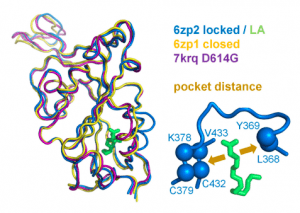
Figure 1: A measure of pocket opening in the RBD. Receptor binding domains are aligned for representative locked (6zp2) and closed (6zp1) (Xiong et al., 2020) S protein trimers, and also for an S trimer structure carrying the D614G mutant, 7krq (Zhang et al., 2021a), with all RBDs down and monomer burial approaching the locked form. Distance across the pocket is shown schematically for 6zp2, with linoleic acid (LA) bound. The distance is calculated between the average of the 4 β-strand Cα atoms displayed (left of LA, which align well structurally between these RBDs), and the average of the two Cα atoms shown to the right of LA, present on a turn within a sub-structure that gates the LA binding pocket (Toelzer et al., 2020).
Following analysis of mutations around the LA binding RBD pocket in the Omicron S protein, the findings were suggesting that changes may occur in the locked conformation accessibility at a neutral pH, without LA binding. In addition, it was found that ionizable group changes may contribute to an altered pH-dependence beyond that which is associated with D614G.
In addition to the main hypothesis of this study, the reserahcer further investigated the role of Asp/Glu sidechains on observed pH-dependent effects and histidines H49 and H519 mediating pH dependence.
NB to note: bioRxiv is a preprint server which publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, or guide clinical practice or treated as established information.
Journal article: Warwicker, J., 2021. Relationship between monomer packing and receptor binding domain pocket status in the spike trimer of SARS-CoV-2 variants. bioRxiv.
Summary by Stefan Botha










