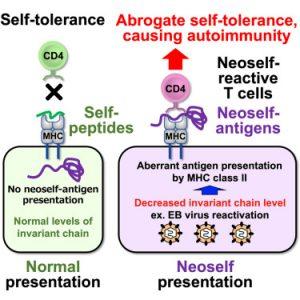
Autoimmune diseases, characterized by the immune system’s erroneous attack on its own tissues, have long puzzled researchers. A recent study published sheds light on this complex phenomenon, suggesting that the body’s own proteins with unusual structures can trigger a cascade of inflammation leading to autoimmunity (Figure 1).
The study focused on the role of the invariant chain (Ii) in the presentation of antigens to T cells. Ii is a crucial component of the major histocompatibility complex II (MHC-II), a protein complex involved in immune responses. When Ii is missing, MHC-II can present larger, misfolded self-antigens, known as neoself-antigens, to T cells.
The researchers found that these neoself-antigens are frequently recognized by T cells in patients with autoimmune diseases, such as lupus. In mice with depleted Ii, the induction of neoself-antigens led to the development of lupus-like symptoms, confirming their role in triggering autoimmune responses.
Furthermore, Epstein-Barr virus (EBV) infection was found to contribute to the presentation of neoself-antigens by downregulating Ii expression. This mechanism could explain why EBV reactivation is associated with the onset or exacerbation of lupus.
The study’s findings challenge the traditional understanding of T cell self-tolerance, demonstrating that T cells can discriminate between self and neoself-antigens. When neoself-antigens are presented on MHC-II, the immune system can mistakenly recognize them as foreign and launch an attack.
This groundbreaking research offers valuable insights into the causes of autoimmune diseases and provides a potential target for the development of novel therapeutic strategies. By understanding the role of neoself-antigens in triggering autoimmune responses, scientists may be able to develop treatments that can prevent or mitigate these debilitating conditions.
Journal article: Mori, S., et al., 2024. Neoself-antigens are the primary target for autoreactive T cells in human lupus. Cell.
Summary by Stefan Botha