Chronic, low-grade inflammation is a defining feature of obesity and a major contributor to diseases such as type 2 diabetes, fatty liver disease, cardiovascular disorders, and cancer. New research reveals a previously unrecognized metabolic mechanism that explains why immune cells in obesity become persistently overactive (Figure 1).
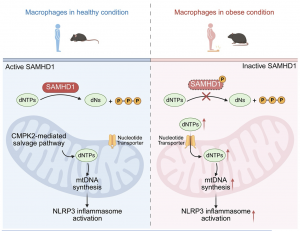
Figure 1: Obesity increased SAMHD1 phosphorylation and thereby compromised its deoxynucleoside triphosphatase function, ultimately leading to aberrant accumulation of cytosolic dNTPs in macrophages. These dNTPs are then transported into mitochondria through nucleotide transporters to provide excessive building blocks for new mtDNA synthesis, resulting in the bypass of CMPK2-mediated nucleotide salvage pathway, ultimately leading to uncontrolled mtDNA neosynthesis, overproduction of ox-mtDNA, and subsequent NLRP3 inflammasome hyperactivation.
The study shows that macrophages from obese individuals and animal models exhibit heightened activity of the NLRP3 inflammasome, a molecular complex that drives inflammatory signalling. This overactivation leads to excessive production of the inflammatory cytokine IL-1β, which is known to worsen insulin resistance and metabolic dysfunction.
At the core of this process is altered nucleotide metabolism. In obese macrophages, levels of deoxynucleoside triphosphates (dNTPs), the building blocks of DNA, are abnormally high. This occurs because the enzyme SAMHD1, which normally breaks down excess dNTPs, becomes functionally impaired. As a result, surplus dNTPs accumulate and are transported into mitochondria, fuelling excessive mitochondrial DNA replication and oxidation.
The buildup of oxidized mitochondrial DNA acts as a danger signal that strongly activates the NLRP3 inflammasome, locking macrophages into a hyperinflammatory state. Importantly, blocking the transport of dNTPs into mitochondria was sufficient to reduce inflammasome activation in immune cells from obese humans and animal models.
These findings identify SAMHD1 as a critical brake on inflammation that operates across species, from fish to humans. They also point to mitochondrial nucleotide transport as a promising therapeutic target to reduce obesity-driven inflammation and slow the progression of related metabolic diseases.
Journal article: Liu, D., et al. 2026. Nucleotide metabolic rewiring enables NLRP3 inflammasome hyperactivation in obesity. Science.
Summary by Stefan Botha










