During B-cell development within the germinal centre, B-cells can go through multiple rounds of both proliferation and mutation. Some of the mutations these B-cells accumulate are taken to possibly contribute to malignant transformation. Over expression of BCL-6, one of the key promoters responsible for GC development has actually been linked to the process of pathogenesis of diseases like diffuse large B cell lymphoma (DLBCL).
Since CD4 T-cells do have a role to play in anti-tumor immunity, the authors sought to find out what role CD4 T-cells play when it comes to aiding the tumour-suppressing function of CD8 T-cells, and possibly unpack the exact mechanisms that can account for the immunosurveillance observed from CD4 T-cells.
One aspect that was looked at was the role of CD137L, that has been proposed to function as a lymphoma suppressor via regulating GC B-cell responses. This was done using a well-established B-cell lymphoma mouse model, the Iμ-Bcl6Tg/+ strain. To shorten the time of lymphomagenesis, they crossed Iμ-Bcl6Tg/+ and the Cd8-/- mice (Iμ-Bcl6Tg/+Cd8-/-), since CD8 T-cells are important for the immuno-surveillance of B lymphoma cells in the Iμ-Bcl6Tg/+ strain.
As seen in Figure 1c, blocking CD137L significantly increased the fraction of cells with an activated B cell phenotype in the Iμ-Bcl6Tg/+Cd8-/- mice. Purified B cells from 12-week-old WT or disease-free Iμ-Bcl6Tg/+ mice were cultured in vitro in the presence of an anti-CD40 antibody, IL-4, and IL-21 to induce a GC phenotype. In figure 1d and 1e, we see that crosslinking CD137L by the addition of recombinant CD137 protein to the culture increased the differentiation of Iμ-Bcl6Tg/+ B cells into GC-like cells. Based on this data, the authors suggest that CD137/CD137L-mediated signalling in Iμ-Bcl6Tg/+ B cells promotes activated B cells to differentiate into GC cells.
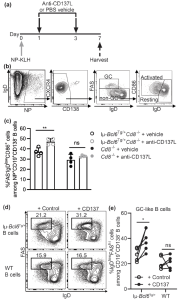
Figure 1: CD137-CD137L interactions promote the differentiation of CD86hiIgD lo premalignant cells into GC cells in Iμ-Bcl6Tg/+Cd8-/- mice. “(a) Iμ-Bcl6Tg/+Cd8-/- or Cd8-/- mice were immunised with 100 μg NP-KLH in alum on day 0 (n = 4 for each group). On day 1 and 3, mice were given 200 μg anti-CD137L blocking antibody or vehicle i.p. before spleens were harvested on day 7. (b) Representative gating strategy for NP+ naive, activated, and GC B cells among CD19+CD138- B cells. (c) Quantification of percentages of NP+ activated and GC B cells among CD19+CD138- B cells of all groups. An unpaired t-test was used. (d) Representative gating strategy for IgDlowFAShi GC-like B cells after 3.5 days culture in plates coated with or without recombinant mouse CD137 protein. (e) Quantification of IgDlowFAShi GC-like B cells among total CD19+CD138- B cells as gated in d after culture.
In their next set of experiments, they were able to determine that blocking CD137L in vivo promoted B-cell lymphoproliferative disease in their mouse model. Based on this development and the fact that CD137 is primarily expressed on activated T cells, the authors raised a hypothesis of CD 4 T-cells possibly preventing the onset of B-cell malignancies via a CD137-CD137L axis.
In figure 2, RNA sequencing of 12 lymphomas derived from T cell-sufficient mice (Iμ-Bcl6Tg/+) and 11 from T cell-deficient mice (Iμ-Bcl6Tg/+Cd3e-/-) showed that B lymphoma cells that developed in the absence of T cells expressed higher amounts of mRNA corresponding to T-cell costimulatory molecules including CD137L and CD70 than lymphoma cells derived from T-cell sufficient Iμ-Bcl6Tg/+ mice.
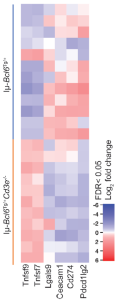
Figure 2: Gene expression of T-cell costimulatory molecules including CD137L is upregulated in B lymphoma cells from Iμ-Bcl6Tg/+Cd3e-/- mice compared to that in B lymphoma cells from T-cell sufficient Iμ-Bcl6Tg/+ mice. “Heatmap of gene expression of T-cell costimulatory or coinhibitory molecules in B lymphoma cells from Iμ-Bcl6Tg/+ and Iμ-Bcl6Tg/+Cd3e-/- mice”.
From the total workup of results, the authors had this to say about their work; “Here we show that the CD137/CD137L costimulatory pathway, also acting in the cognate interactions between premalignant B cells and CD4 T-cells, mediates the further differentiation of BCL6-driven premalignant B cells facilitated by CD4 T cells and this results in the prevention of malignancy at an early activated B cell stage”.
Journal article: Ding, Z., et al., 2022. CD137L and CD4 T cells limit BCL6 ‐expressing pre‐germinal center B cell expansion and BCL6 ‐driven B cell malignancy. Immunology & Cell Biology.
Summary by Vanessa Muwanga










