Immunotherapies, particularly those that block molecules like CTLA-4 and PD-1 have greatly impacted how cancer is treated. We know for a fact that this occurs as a result of these check-point inhibitors enhancing CD8 T-cell responses. What is yet to be understood further is what effect these immunotherapies have on other immune cells types.
In this paper, the authors sought to find what effect anti PD-1 immunotherapies have on the Tfh–B cell axis. To study this, they had a look at the immune responses induced by the influenza vaccine. Investigations like these also add to the body of work on the role that protective vaccines have when used on individuals with cancer that are also receiving immune-modulating medication.
Some major findings from the study include the generation of evidence that show that an increase in germinal centre activity was associated with proliferation of circulating T follicular helper cells (cTfh) as well as B-cells (Figure 1) . The increase in Tfh and B cell responses in the context of anti-PD1 therapy would point to the possibility of the Tfh-B cell axis being another mechanism through which immunotherapies work, aside from boosting CD8 T-cell responses.
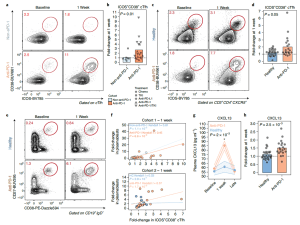
Figure 1: “Anti-PD-1 treatment is associated with enhanced cTfh, B cell and GC responses following influenza vaccination. a, cTfh were profiled for expression of ICOS and CD38 at baseline and at 1 week after influenza vaccine in Cohort 1. b, ICOS+CD38+ cTfh fold-change at 1 week in Cohort 1 (P = 0.01, two-sided Wilcoxon test; anti-PD-1 (n = 18) versus non-anti-PD-1 (n = 7)). c, Patients with melanoma were recruited in Cohort 2 and profiled following influenza vaccination. cTfh responses shown at baseline and 1 week. d, ICOS+CD38+ cTfh fold-change at 1 week compared with baseline in Cohort 2 (P = 0.05, two-sided Wilcoxon test; anti-PD-1 (n = 20) versus healthy (n = 27)). e, Plasmablast responses after influenza vaccination in Cohort 2. f, Kendall correlation between plasmablasts and ICOS+CD38+ cTfh at 1 week for Cohorts 1 and 2. g, Plasma CXCL13 over time (P = 2 × 10−3 for anti-PD-1 at 1 week compared with baseline, two-way ANOVA with Tukey’s post-test). Data are presented as mean value (point) ±s.e.m. (shaded area). h, Plasma CXCL13 fold-change at 1 week in Cohort 2 (P = 2.5 × 10−3, two-sided t-test; anti-PD-1 (n = 20) versus healthy (n = 27)). HC, healthy controls; TKI, tyrosine kinase inhibitor.”
Transcriptional profiling was done on cTfh after vaccination and the authors did identify the presence of altered pathways, some of which include a reduction in IL-2/STAT5 signaling. In the context of anti-PD-1 therapy, the authors take these changes to be associated with a very strong antibody response after vaccination when looked at quantitatively. From a qualitative standpoint however, these antibody responses were seen as being impaired.
The authors do call for more work to be done when it comes to alternative vaccine strategies so that patients on anti-PD-1 treatment are optimally protected. The authors do caution that this is a necessary investigation to carry out and not assume that responses generated after influenza vaccination, for individuals on anti-PD-1 treatment are similar to those that are not on this form of therapy.
A look at cTfh from participants with irAEs versus those without them showed that the former did have hyper-responsiveness to anti-PD-1. The authors take these findings to be in line with other results within the literature that have surprisingly shown more robust T-cell responses and better survival even for several patients with irAEs that were on anti-cancer treatment.
The authors had this to say in summary; “these studies not only reveal a role for treatment with anti-PD-1 to reset the immune landscape beyond anti-cancer responses in some patients, but also suggest the potential of PD-1 pathway manipulation in anti-vaccine responses, and point to activated cTfh including in the context of an analytical vaccine to identify patients at risk of irAEs. The data presented here demonstrate the utility of immunoprofiling studies during a vaccine-induced perturbation as a means of uncovering mechanisms of immunomodulator therapy, which will have major implications for design and use of immunomodulator therapy”.
Journal article: Herati, R.S., et al., 2022. PD-1 directed immunotherapy alters Tfh and humoral immune responses to seasonal influenza vaccine. Nature Immunology.
Summary by Vanessa Muwanga










