There are approximately 38 million individuals who are infected with human immunodeficiency virus (HIV) globally. Advances in HIV care and management, primarily due to the development of safe highly active antiretroviral therapy and ambitions 90-90-90* treatment target has made HIV-infection a very manageable chronic infection. Unfortunately, the successful provision of HAART only results in viral suppression and does not lead to a functional cure. Thus the HIV community is still working hard to develop an efficacious preventive and prophylactic HIV vaccine.
Clinical studies that aim to understand HIV-immunity in HIV infected individuals have identified broadly neutralising antibodies (bnAbs) that bind highly to HIV epitopes. Researchers have shown that these bnAbs are capable of providing sterilising immunity using humanised-murine and non-human primate models. Unfortunately, few individuals naturally develop bnAbs, therefore developing a vaccine that can induce these Abs is one of the goals of HIV vaccinology. A recent study by Huang et al., utilised CRISPR-Cas9 technology and immunisation to induce HIV bnAbs in humanised mice.
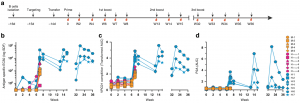
Serological analysis of VRC01 responses after vaccination. a Engineered B cell vaccine experimental design. Time course of B cell engineering, cell transfer, immunization, and blood sampling in WT C57BL/6J mice. b–d Serum antibody responses after MD39-ferritin immunization in mice that received mock targeted, H-targeted, or H + κ targeted B cells. Titers (y-axis) are given for the indicated timepoints (x-axis) and mice (legend): b Total (host + donor) IgG response to the immunogen, given as serum dilution factor (SDF) EC50s; c VRC01-competetive antibody responses given as the transformed area under the curve (AUC) and; d Serum IgG carrying the P2A tag on engineered L-chains, given as area under the curve (AUC). (Source: Huang et al., 2020)
They isolated mature B cells from C57BL/6J mice and utilised CRISPR-Cas9 to modify B cells to express VRC01 (Ab that binds the HIV CD4 receptor binding site) B cell receptor. Modified B cells were then transferred to naive mice. Mice (including controls) were then vaccinated with MD39 (antigen that has a monomeric affinity for VRC01)-ferritin, a soluble stabilised HIV envelope native trimer multivalently presented on ferritin based nanoparticle in a prime-(3) boost manner. Experimental protocols demonstrated that mice that received VRC01-engineered B cells developed 10-fold higher antigen-specific Ab titres than controls mice (who were also vaccinated). Further, VRC01-engineered B cells were capable of undergoing class-switching, affinity maturation and somatic hypermutation suggesting that these B cells can respond to pathogen evolution. Researchers concluded that “these results encourage further exploration of engineered B cell vaccines as a strategy for durable elicitation of HIV bnAbs to protect against infection and as a contributor to a functional HIV cure.”
Journal Article: Huang et al., 2020. Vaccine elicitation of HIV broadly neutralizing antibodies from engineered B cells. Nature Communication
*90-90-90 treatment target: Goal aims to ensure that 90% of all people living with HIV will know their HIV status of which 90% of diagnosed individuals should receive HAART (regardless of CD4 count). Further, 90% of all people receiving antiretroviral therapy should have viral suppression.










