In this study, Ebenig and colleagues were able to come up with a detailed description (figure 1) of what they believe to be the immune-cell mediated mechanisms responsible for inducing Vaccine-associated enhanced respiratory disease (VAERD). VAERD is taken to be one of the most complex and severe immune reactions that can occur after one has a respiratory infection. With the disruption that COVID brought about, the quickened development and almost immediate rollout of several potential vaccine candidates was warranted. The authors do caution however, that the full effects of all vaccinations, always need to be looked at and understood.
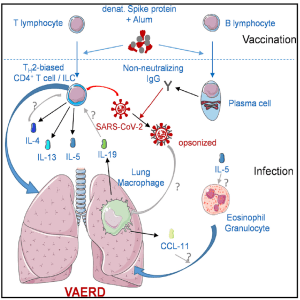
Figure 1: Graphical abstract to summarise the paper: Ebenig et al. report vaccine-associated enhanced respiratory disease (VAERD) in SARS-CoV-2-infected Syrian hamsters previously immunized with a suboptimal vaccine consisting of misfolded SARSCoV- 2 spike protein and Alum adjuvants. VAERD is correlated with dysregulation of specific immune cell subsets in the lungs and could be alleviated by dexamethasone treatment.
The model they propose (figure 1) for the induction of VAERD comes as a result of vaccination with a suboptimal Th2-biased prototypic vaccines created to target SARS-CoV-2. The particular investigation into VAERD comes as a result of other literature that shows the induction of VAERD in other animal models where vaccination against CoV occurred.
Ebenig et al were able to carry this out by delivering a sub-optimal immunization dose of alum-adjuvanted, non-stabilized S protein to Syrian hamsters subcutaneously (figure 2). The authors noted that the only binding antibodies that were induced were those with no neutralizing activity, and that they observed no killing activity from antigen-specific CD8+ T cells. The results from this study, however, are not taken to be very surprising as the authors do state that they expected to see a mediocre immune response that was biased towards the TH2 phenotype because of this vaccination strategy.

Figure 2: “Schematic depiction of experiment. Syrian hamsters were bled and subsequently immunized with indicated vaccines on days 0 and 21 (n = 6/cohort). On day 35, immunized hamsters were bled and intranasally challenged with low-passage SARS-CoV-2 and observed for another 4 days before sacrifice for organ preparation”.
The authors note that only two other studies (DiPiazza et al, 2021, Iwata-Yoshikawa et al, 2022) so far, have been in position to provide evidence for the potential of VAERD occurring as a result of vaccination of TH2-prone BALB/c mice with denatured antigen after exposure to a mouse-adapted recombinant SARSCoV-2.
One limitation they cite is the inability to extrapolate the results done in animal models to human beings and thus make strong conclusions about what may fully result if this were to happen in the human body. In spite of this, this is what the authors did have to say about their work; “our data strongly support the idea of monitoring vaccinated human patients that experience a break-through infection closely. In any case, while our experimental vaccines mimic, but are not the same as, the authorized vaccines, these and previously published data point to few concerns for vaccines developed to trigger TH1-biased responses such as viral vector platform-based vaccines, mRNA vaccines, or other vaccine concepts developed for such bias”.
Since they were able to demonstrate that VAERD can be treated with dexamethasone, the authors mention that if this form of immunopathology were to occur and be observed in human beings, then VAERD could be treatable using dexamethasone, which is a drug that has already been shown to a useful medication for severe courses of COVID-19.
Journal article: Ebenig, A. et al., 2022. Vaccine-associated enhanced respiratory pathology in COVID-19 hamsters after TH2-biased immunization. Cell reports.
Summary by Vanessa Muwanga










