Our bodies naturally produce a powerful molecule called interleukin-15 (IL-15), a growth factor critical to the immune system’s ability to detect and eliminate cancer cells. IL-15 stimulates the production and activity of key immune defenders, including natural killer (NK) cells—so named for their ability to swiftly target and destroy abnormal cells without prior sensitization.
However, cancer cells are adept at evading this immune surveillance. Despite producing IL-15 themselves, tumours often suppress NK cell responses through various mechanisms, tipping the balance in favour of disease progression.
One proposed solution has been to administer drugs that activate the IL-15 receptor on immune cells to restore anti-tumour immunity. But this approach has proven too toxic—because it globally stimulates immune cells throughout the body, not just in the tumour—leading to serious systemic side effects.
Now, researchers have identified a novel workaround. By using CRISPR-based screening, they discovered a gene that, when switched off in NK cells, dramatically increases their sensitivity to IL-15—even at low levels (Figure 1).
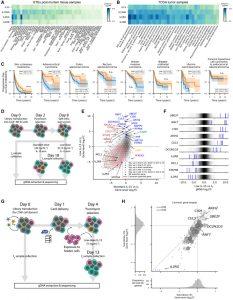
Figure 1 Identification of dominant IL-15R regulators. (A and B) Analysis of IL15, IL15RA, IL2RG, and IL2RB in (A) GTEx postmortem tissue, (B) TCGA tumor tissue samples. (C) Kaplan-Meier plots of progression free survival probability based on IL15 transcript abundance for TCGA cohorts. Tumors with low (blue; bottom 30%) or high (orange; top 30% IL15 expression) are shown. Significance of the survival differences between low and high IL15 was assessed using log rank test. (D) Schematic of genome-wide NK92 CRISPR screen in low/non-mitogenic (0.15 ng.mL−1) IL-15 or saturating (20 ng.mL−1) IL-15 concentrations. (E) Gene-level differential abundance of sgRNAs from NK92 CRISPR screens under low/non-mitogenic or saturating IL-15 concentrations (relative to t0), showing significantly enriched and select depleted sgRNAs (FDR <0.1). (F) Guide-level differential abundance under low/non-mitogenic IL-15 (relative to t0), where the four individual gRNAs (blue vertical lines) for selected genes are shown relative to all other sgRNAs (grayscale density). (G) Schematic of the sublibrary primary human NK cell CRISPR screens in 5 ng.mL−1 IL-15 with cancer target cell co-culture; n = 3 independent donors. (H) Gene-level differential abundance of common sgRNAs shared across two sub-libraries from primary human NK cell CRISPR screens, with matched histograms showing the distribution of gene level differential gRNA abundance for each sublibrary (rs = Spearman’s correlation, rp = Pearson’s correlation). (ns, not significant; ∗p < 0.05; ∗∗< 0.01; ∗∗∗∗< 0.0001).
The gene in question encodes an enzyme that appears to act as a brake on IL-15 responsiveness. Disabling it made NK cells much more reactive to the body’s natural IL-15, which is found in higher concentrations in tumours such as colorectal cancer. When tested in preclinical models, this gene modification enhanced NK cell function and significantly slowed tumour growth.
What makes the discovery especially promising is the therapeutic potential: enzymes are highly “druggable” targets. In fact, a compound that incidentally blocks this same molecular pathway has already shown anti-cancer activity in early trials for myelodysplastic syndrome, suggesting that more selective and safer inhibitors could be developed.
Unlike systemic IL-15 therapies, this strategy could allow localized immune activation where it’s needed most—within tumours—while sparing healthy tissues where IL-15 levels are minimal. Further bolstering its potential, the gene-modulating approach is compatible with existing immunotherapies, such as checkpoint inhibitors.
With two candidate genes now identified—both of which could be deleted in engineered cell therapies or targeted with small-molecule drugs—the research offers a new path to enhancing the innate immune system’s fight against cancer.
Journal article: I. Nikolic, et al., 2025. Enhancing Anti-Tumor Immunity of Natural Killer Cells through Targeting IL-15R Signaling, Cancer Cell.
Summary by Stefan Botha










