New drug compounds are tested for safety and effectiveness in animal models before being approved for clinical trials in humans, whereby, for example, rats and mice have been used extensively in clinical research to test various compounds and for drug discovery.
Recently, researchers began working to develop non-mammalian alternatives that could reduce the number of rodents used in biomedical research and one that could also lower costs and accelerate results. These researchers decided to make use of caterpillars to study gut inflammation -; a risk factor for developing colorectal cancer.
Their research used the larvae of the tobacco hornworm (Manduca sexta) because they have a high degree of similarity to the human gut structure and physiology -; what scientists call a high degree of evolutionary conservation. Tobacco hornworm caterpillars are large enough to be imaged with the same instruments used for human patients (computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) scans) (Figure 1). Caterpillars are basically just one long bowel, so they made a great model for studying inflammatory bowel disease.
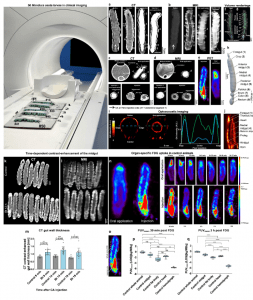
Figure 1: Experimental setup and validation of multimodal imaging of Manduca sexta larvae. 50 Manduca sexta larvae were imaged in a clinical MR scanner. The midgut of the M. sexta larvae was imaged by CT (a, c, h), MRI (b, d, f, g), PET (e), and OAT (i and j: Experiments were repeated independently: n = 4 with similar results) with or without the injection and/or oral application of a contrast agent (CA). Axial image of the isolated midgut of M. sexta exposed to CA washed out with 0.9% NaCl (c and d, midgut section + application). Volume rendering of the alimentary tract of M. sexta obtained from MRI (f, g) after injection of Gd-BOPTA, or CT (h Experiments were repeated independently: n = 30 with similar results) after oral application of iodixanol. Time-dependent contrast-enhancement of the midgut after injection of CA in animals 12 h after Bt infection. k, l Coronal CT and MR images of control and Bt-infected animals after the injection of CA. m CT contrast-enhanced gut wall thickness of control and Bt-infected animals 12 h after Bt infection at 9 min, 14 min, and 19 min after CA injection. The most significant difference between control and Bt infected animals was 14 min after CA injection, n = 18, two-tailed t-test. n FDG-PET images after oral application or intracardial injections and time-dependent uptake of FDG. o Area of PUVmaxn determination. Organ-specific FDG uptake in control animals. PUVmaxn values of whole larvae and isolated organs after (p) 39 min and (n = 63, Kruskal-Wallis-test = 55.1 4, P= < 0.0001, box plots: 25th–75th percentiles, whiskers: min-max (show all points), center: median) (q) 3 h (n = 50, one-way ANOVA, F(4,45) = 501.0, R2 = 0.978, P < 0.0001, box plots: 25th–75th percentiles, whiskers: min–max (show all points), center: median) of FDG application are shown. The following significance levels have been used: ns = P > 0.05, * = P ≤ 0.05, ** P ≤ 0.01, *** = P ≤ 0.001 and **** = P ≤ 0.0001. Bar charts represent mean and SD. Every data point represents a single animal. Source data are provided as a Source Data file.
As such, there are a lot of standards and policies that have been developed around the use of animals in research and their care. Starting a new experiment in a mouse model, just even trying another drug combination, can take several months to add it to a protocol and get it approved.
Since caterpillars are invertebrates, one faces less administrative burden. One can just design the experiment and then do it, significantly speeding up the research. In addition, mammals are very slow-growing and expensive to house compared with invertebrates. So these experiments can be done at a lot less cost.
Journal article: Windfelder, A.G., et al., 2022. High-throughput screening of caterpillars as a platform to study host-microbe interactions and enteric immunity. Nature Communications.
Summary by Stefan Botha










