In a recent study, researchers have unveiled a double-acting strategy for combating the notoriously aggressive and challenging-to-treat triple-negative breast cancer (TNBC) (Figure 1). Their findings showcase the potential of silencing a specific gene, ACSS2, to significantly enhance the effectiveness of existing treatment methods.
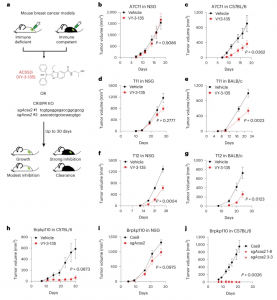
Figure 1: Inhibition of ACSS2 triggers stronger tumor growth inhibition in immune-competent mice compared to immune-deficient mice. a, Diagram outlining the approach to testing inhibition of ACSS2 in immune-competent and immune-deficient tumor-bearing mice. VY-3-135, ACSS2 inhibitor; sgAcss2, single-guide RNA targeting Acss2; NSG, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ. b–g, Allograft tumor growth in NSG, C57BL/6 or BABL/c mice treated with VY-3-135. n = 8 mice per group. Adjusted P values are displayed on the graph and were generated using a multiple two-sided Welch t-test. Values represent the mean tumor volume ± s.e.m. h, Orthotopic allograft tumor growth of Brpkp110 cells in C57Bl/6 mice treated with VY-3-135. Adjusted P value is displayed on the graph generated using multiple two-sided Welch t-test. Values represent the mean tumor volume ± s.e.m. n = 5 mice per group. i,j, Orthotopic allograft tumor growth of Cas9 (WT) or two different sgAcss2 (Acss2-KO) Brpkp110 cell lines in NSG or C57BL/6. Adjusted P values are displayed on the graph and were generated using multiple unpaired two-sided Welch t-test. Values represent the mean tumor volume ± s.e.m. NSG, n = 10 mice per group. C57BL/6, n = 7 mice per group for Cas9 and n = 5 mice per group for each Acss2-KO.
Triple-negative breast cancer poses a formidable challenge as it lacks three crucial receptors – estrogen, progesterone, and HER2 – that can be targeted with precision in other forms of breast cancer. This receptor absence leaves limited options for treating TNBC, which affects 10-15% of breast cancer patients in the US.
This study introduces an innovative double-acting concept: by silencing the ACSS2 gene, TNBC metabolism is disrupted, and simultaneously, the immune system’s ability to combat the cancer is bolstered. ACSS2 plays a pivotal role in regulating acetate, a nutrient that TNBC cells exploit for their growth and spread. The researchers employed two methods for deactivating ACSS2: CRISPR-Cas9 gene editing and a potent ACSS2 inhibitor known as VY-3-135.
Targeting ACSS2 in preclinical experiments not only hindered TNBC’s ability to metabolize acetate, thus stunting its growth, but it also prompted the immune system to recognize and attack the cancer. Strikingly, the immune system exhibited an ability to exploit acetate that TNBC cells couldn’t process. What’s more, this immune response was so effective that it established a memory of how to combat TNBC in the future, even when the tumour’s ACSS2 gene remained active.
These findings herald a new ray of hope for TNBC patients. Silencing ACSS2 represents a promising avenue for disrupting the cancer’s growth while harnessing the immune system’s formidable abilities to fight it. This novel double-acting approach offers a potential breakthrough in the treatment of this aggressive form of breast cancer, providing a brighter outlook for patients facing this formidable adversary.
Journal article: Miller, K.D., et al. 2023. Acetate acts as a metabolic immunomodulator by bolstering T-cell effector function and potentiating antitumor immunity in breast cancer. Nature Cancer.
Summary by Stefan Botha










