The need to find treatments for COVID-19, caused by the novel coronavirus 2 (CoV-2), has dramatically accelerated. Drugs that stop the severe acute respiratory syndrome (SARS) caused by CoV-2 could save the lives of severely ill patients, protect health care workers and others at high risk of infection, and reduce the time patients spend in hospital beds.
The World Health Organization (WHO) has announced the start of a major study that will compare treatment strategies, built as a streamlined clinical trial, which doctors around the world can join. The plan is to test at least 12 potential COVID-19 treatments, including drugs already in use for HIV and malaria, experimental compounds that work against an array of viruses in animal experiments, and antibody-rich plasma from people who have recovered from COVID-19.
In an attempt to avoid repeating the mistakes of the 2014–16 West African Ebola epidemic, where clinical trials were set up late, leading to the under-recruiting of patients – in the current pandemic researchers have decided to start trials as soon as possible so they can rapidly have the most efficacious interventions come to the front.
On the 20th of March 2020 WHO announced the launch of SOLIDARITY, an unprecedented, coordinated trial designed to collect robust scientific data rapidly during the COVID-19 pandemic. In the study, which has the potential to include thousands of patients in dozens of countries, simplicity if emphasized, so that even hospitals overwhelmed by an onslaught of COVID-19 patients can participate. Using WHO’s website patients will be randomized to local standard care or one of the four drug regimens tested in the first stage of the trial, depending on what is available at the patient’s hospital. Physicians will simply record the day the patient left the hospital or died, the duration of the hospital stay, and whether the patient required oxygen or ventilation.
To note, the design of the study is not blinded; as patients will know whether they received a drug candidate, this might give rise to a potential placebo effect. In terms of the drugs tested, the WHO will repurpose drugs that are already approved for other diseases and have acceptable safety profiles. Additionally, they will include experimental drugs that have performed well in animal studies against two other deadly coronaviruses that cause SARS and Middle East respiratory syndrome (MERS).
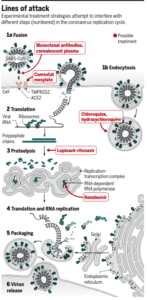
More specifically, the following four treatments will be tested: the experimental antiviral drug remdesivir, the malaria medication chloroquine (or its alternative hydroxychloroquine), a combination of the HIV drugs lopinavir and ritonavir, and the lopinavir-ritonavir combination with an addition of interferon-beta, an immune system messenger that helps cripple viruses. Notably, although these treatments will hopefully stop the virus by different mechanisms, each one of the four has drawbacks.
In addition to these, other approved and experimental treatments against coronavirus are currently being or soon likely to be tested by researchers. These include drugs that reduce inflammation, such as corticosteroids and the rheumatoid arthritis drug baricitinib. Some researchers have high hopes for camostat mesylate, a drug licensed in Japan for pancreatitis. Other antivirals will also likely be tested, including the influenza drug favipiravir and additional HIV antiretrovirals. Additionally, researchers plan to try boosting immunity with “convalescent” plasma from recovered COVID-19 patients or monoclonal antibodies directed at SARS-CoV-2.
The overall purpose of the trial is to test the drugs in people during early stages of the disease, who doctors think are most likely to get much worse.
Reference
Kupferschmidt, K., & Cohen, J. (2020). Race to find COVID-19 treatments accelerates. Science
Article by Naffesa Al Sheikh