A new proposes a promising strategy to protect insulin-producing cells and potentially delay or prevent Type 1 diabetes (Figure 1). By blocking a key inflammation-related protein, TYK2 (tyrosine kinase 2), researchers were able to reduce immune system attacks on the pancreas in preclinical models. Crucially, a TYK2 inhibitor is already FDA-approved for treating psoriasis, which may accelerate the path toward clinical testing for diabetes.
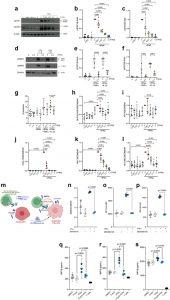
Figure 1: TYK2 inhibitors repress IFNα signalling and inflammatory gene expression and reduce β cell immunogenicity. Dispersed human islet cells were allowed to rest in culture for two days before pre-treatment for 2 h with the indicated concentrations (expressed in mM) of TYK2i BMS-986165. Treatment was continued with BMS-986165 in the absence or presence of IFNα (2000 U/mL), IFNα (2000 U/mL) + TNFα (1000 U/ml), or IFNα (2000 U/mL) + IL-1β (50 U/mL) for 24 h. (a–f) Western blotting of protein lysates was performed to detect phospho-STAT1/2. Data were normalised to the levels of β-actin and expressed relative to treatment with IFNα alone. (g) Apoptotic cells were identified by Hoechst 33342 and propidium iodide staining. (h–l) Total RNA was extracted and mRNA levels of (h) DDIT3 (CHOP), (i) ATF3, (j) CXCL10, (k) MX1, and (l) HLA-ABC were analysed by RT-qPCR. (m) Schematic representation of the experimental workflow on EndoC-βH1/HLA-A2 cells co-cultured either with HLA-A2 alloreactive or autoreactive CD8+ T cell clones directed against insulin defective ribosomal product and/or preproinsulin specific T cells. (n) RT-qPCR analysis showing B2M expression in EndoC-BH1/HLA-A2 cells upon IFNα treatment (2000 U/mL) in the presence or absence of 1 uM BMS-986165. (o) Surface expression of HLA-ABC and (p) HLA-A2 in EndoC-βH1/HLA-A2 cells prior to co-culture. Cells were exposed to IFNɑ in the presence or absence of BMS-986165 for 24 h. T cell activation assay (MIP-1β release) after co-culture with (q) A2 alloreactive T cells, (r) autoreactive T cells directed against insulin defective ribosomal product, or (s) preproinsulin specific T cells. For RT-qPCR analysis, expression data were normalised by the geometric mean of GAPDH and ACTB expression levels and expressed relative to cells exposed to IFNα alone. Statistical analyses for human islet studies (n = 3–7) were conducted using one-way ANOVA with multiple comparisons between treatment groups. Co-culture experiments were performed with independent biological replicates (n = 3). An unpaired t-test (with or without Welch’s correction) was applied to evaluate the expression of surface proteins (panels n, o, and p), while one-way ANOVA with multiple comparisons was used to analyse differences between treatment conditions (all remaining panels excluding a, d, and m). All data are presented as the mean with 95% confidence intervals (CI).
Key Highlights
- Type 1 diabetes (T1D) results from the immune system attacking pancreatic beta cells, which produce insulin.
- Researchers used human cells and mouse models to block TYK2 signaling, a driver of inflammation in T1D.
- This reduced pancreatic inflammation and protected beta cells from immune attack.
Why TYK2?
- TYK2 is a key regulator of cytokine signaling—chemical messengers that promote inflammation.
- People with naturally lower TYK2 activity are less likely to develop T1D, supporting its role as a therapeutic target.
- A TYK2 inhibitor drug is already approved for psoriasis, an autoimmune skin disease, offering a repurposing opportunity.
Implications
- Could delay disease onset in high-risk individuals (e.g., those with islet autoantibodies).
- Offers a dual-action therapy: protect beta cells + reduce autoimmune inflammation.
- Builds on real-world safety data from the psoriasis indication.
This research adds momentum to efforts aiming not just to manage but to prevent Type 1 diabetes, using immune-modulating therapies already available in clinical practice.
Journal article: Syed, F., et al. 2025. Pharmacological inhibition of tyrosine protein-kinase 2 reduces islet inflammation and delays type 1 diabetes onset in mice. eBioMedicine.
Summary by Stefan Botha










