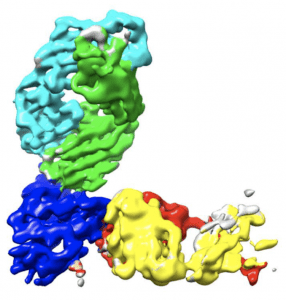
Complex of REGN10933 and REGN10987 with the SARS-CoV-2 RBD. (A) 3.9 Å cryoEM map of REGN10933 + RBD + REGN10987 complex, colored according to the chains in the refined model (B). RBD is colored dark blue, REGN10933 heavy and light chains are green and cyan, and REGN10987 heavy and light chains are yellow and red, respectively. (Source: Hansen et al., 2020)
Using a humanized mouse model and B cells from COVID-19 convalescent individuals, Hansen et al., describe the discovery of two SARS-CoV2-specific antibodies: REGN10933 and REGN10987 that are currently being tested for immunotherapeutic potential against COVID-19 (NCT04426695; NCT04425629)*. Both antibodies non-competitively bind to SARS-CoV-2 receptor binding domain of the spike protein preventing binding to ACE-2, resulting in neutralisation of the virus. In addition to binding activity mediated by the antibody binding fragment, antibodies also mediated effector functions via the fragment crystallizable region. Some of these effector functions such as antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP), bridge humoral immunity with cellular immunity, contribute to increased clearance of viral-infected cells. Hansen et al., also demonstrated that of all antibodies tested REGN10987 displayed the best ADCC activity and had similar ADCP capacity as REGN10933.
Journal Article: Hansen et al., 2020. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science
Summary by Cheleka Mpande
Note: Clinical trials testing REGN10933 and REGN10987 began in June 2020 and will be tested in COVID-19 patients with different pathological features and clinical care needs.










