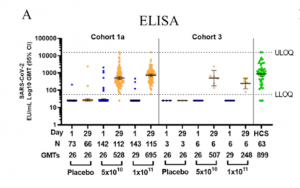
Log geometric mean titers (GMTs – as illustrated by the horizontal bars and the numbers below each timepoint) of SARS-CoV-2 binding antibodies in serum as measured by ELISA (ELISA Units per mL [EU/mL]), at baseline and at Day 29 post vaccination, among all participants, according to schedule in cohort 1a and 3. Dotted lines indicate the lower limit of quantification (LLOQ) and upper limit of quantification (ULOQ) of the assay, error bars indicate 95% confidence interval (CI). For values below the LLOQ, LLOQ/2 values were plotted. (Source Sadoff et al., Pre-print)
A recent pre-peer reviewed article appearing in medRxiv reports on an interim analysis of an adenovirus type 26 SARS-CoV-2 vaccine candidate (Ad26.COV2.S). This was a phase 1/2a randomized, double-blinded, placebo-controlled clinical study consisting of a non-replicating adenovirus 26 based vector expressing the stabilized pre-fusion spike (S) protein of SARS-CoV-2. The vaccine was given at a dose level of 5×1010 or 1×1011 viral particles per vaccination, either as a single dose or as a two-dose schedule spaced by 56 days in just over 800 healthy adults at varying ages. The most frequent local adverse event (AE) was injection site pain and the most frequent solicited AEs were fatigue, headache and myalgia. The seroconversion rate at approximately 1 month after vaccination for either vaccine dose varied from 83-100% depending on the group vaccinated. At either does, the vaccine induced strong neutralizing antibody responses and comparable responses were observed in 18-55 year old versus 65-75 year old adults. Two weeks after immunization, an exclusive Th1 S-specific CD4+ response was observed in 80-83% of participants, with extremely low Th2 responses. There was also a robust CD8+ T cell response at both dose levels. These results show that a single dose of either level was “safe, well tolerated and highly immunogenic”. The authors propose that this candidate moves to phase 3 trials.
Sadoff et al., Pre-Print. Safety and immunogenicity of the Ad26.COV2.S COVID-19 vaccine candidate: interim results of a phase 1/2a, double-blind, randomized, placebo-controlled trial. MedRxiv
Summary by Clive Gray










