BioNTech, a German-based biotech company, recently published a Pre-Print describing results from their Phase 1/2 safety and immunogenicity BNT162b1 COVID-19 vaccine trial#. BNT162b1 is a lipid-nanoparticle encapsulated RNA vaccine that encodes the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. The biotech company incorporated 1-methyl-pseudouridine instead of uridine in to the RNA sequence which dampens innate immune sensing of the RNA sequence and results in increased mRNA translation in vivo.
Researchers compared safety and immunology profiles of 5 different dose levels (1μg, 10μg, 30μg, 50μg and 60ug) of the vaccine in 12 individuals per dose. They initially planned to vaccinate individuals in all groups with two doses of the vaccine, 21 days apart. However, due to increased reactogenicity reported in the 50μg prime-boost group, individuals that received the 60μg dose levels only received on vaccination dose.
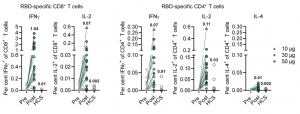
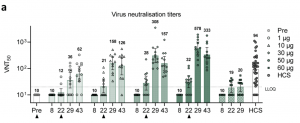 Sahin et al., reported a dose-dependent increase in reported reactogenicity, mild-medium side effects, C reactive-protein and a decrease in blood lymphocyte counts in vaccinees, which resolved quickly. They reported a dose-dependent increase in BNT162b1-specific IgG and neutralisation capacity, which were significantly boosted by the 2nd vaccination. Additionally, vaccine-induced antibody titres that were superior to those detected in COVID-19 convalescent sera. To ensure that vaccine-induced immunity also targeted other SARS-CoV-2 variants, they showed that BNT162b1-specific antibodies could also neutralise 17 other SARS-CoV-2 viral strains including the dominant circulating strain D614G. Finally, they showed that 94.4% and 80.6% of vaccinated individuals induced robust RBD-specific CD4 and CD8 T cells, respectively, determined by IFNγ-ELISPOT. Detectable RBD-specific CD4 T cells predominantly expressed Th1 associated cytokines IFNγ and IL-2 but not IL-4 upon stimulation. Further, no dose-dependent increase in T cell immunity was observed (figure below).
Sahin et al., reported a dose-dependent increase in reported reactogenicity, mild-medium side effects, C reactive-protein and a decrease in blood lymphocyte counts in vaccinees, which resolved quickly. They reported a dose-dependent increase in BNT162b1-specific IgG and neutralisation capacity, which were significantly boosted by the 2nd vaccination. Additionally, vaccine-induced antibody titres that were superior to those detected in COVID-19 convalescent sera. To ensure that vaccine-induced immunity also targeted other SARS-CoV-2 variants, they showed that BNT162b1-specific antibodies could also neutralise 17 other SARS-CoV-2 viral strains including the dominant circulating strain D614G. Finally, they showed that 94.4% and 80.6% of vaccinated individuals induced robust RBD-specific CD4 and CD8 T cells, respectively, determined by IFNγ-ELISPOT. Detectable RBD-specific CD4 T cells predominantly expressed Th1 associated cytokines IFNγ and IL-2 but not IL-4 upon stimulation. Further, no dose-dependent increase in T cell immunity was observed (figure below).
In summary, Sahin et al demonstrated that the BNT162b1 vaccine is safe and immunogenic, inducing superior RBD-specific humoral and cellular immunity than those detected in convalescent patients.
Journal Article: Sahin et al., (Pre-Print). Concurrent human antibody and TH1 type T-cell responses elicited by a COVID-19 RNA vaccine. medRxiv
# Also Read Mulligan et al. 2020. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature
Summary by Cheleka Mpande