A new study has demonstrated that pembrolizumab, an anti-PD-1 immune checkpoint inhibitor, induces remarkable tumour regression in advanced unresectable desmoplastic melanoma (Figure 1). Nearly 90% of patients responded, underscoring pembrolizumab as a highly effective and low-toxicity treatment option for this rare, aggressive melanoma subtype.
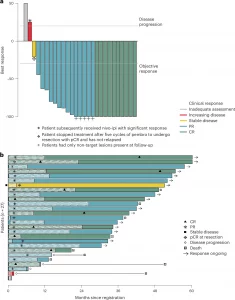
Figure 1: Depth of response and duration of response. a, Waterfall plot demonstrating the best response, as percentage change in RECIST target lesions, to pembrolizumab. The vertical bars represent the largest percentage decrease or, if no decrease was observed, the smallest percentage increase in the size of target lesions against baseline. The lines indicate the threshold for objective response (≥30% decrease) or disease progression (>20% increase). One patient did not have follow-up disease assessment data and is denoted on the far left with a gray bar at 50% over baseline. b, Swimmer plot of patient outcomes, including timing of PRs and CRs and PFS events. The horizontal bars represent time from registration. Shaded regions of the bars represent duration of protocol treatment. Patients with arrows are alive and progression free. The patient represented by the gray bar at the bottom withdrew consent after one cycle of treatment; no follow-up outcome data were obtained. nivo-ipi, nivolumab plus ipilimumab; pembro, pembrolizumab.
Key Findings
- Cohort B of the SWOG S1512 trial enrolled 27 patients with unresectable/metastatic desmoplastic melanoma.
- Treatment protocol: Pembrolizumab every 3 weeks for up to 2 years.
- Clinical outcomes:
- 37% complete response (CR) – full tumour disappearance.
- 89% overall response rate (ORR) – tumour shrinkage or disappearance.
- Responses often occurred within 2 months.
- At 3-year follow-up:
- 84% alive
- 72% progression-free
- Durability: Many patients-maintained remission after stopping therapy.
- Safety: Generally well-tolerated, even in older patients; some discontinued early due to side effects, but efficacy was preserved.
Scientific & Clinical Significance
- Desmoplastic melanoma is characterized by:
- High tumour mutational burden (from chronic UV exposure)
- Increased immune visibility, predisposing to strong PD-1 responses.
- Confirms anti-PD-1 monotherapy as the preferred first-line option in this melanoma subtype, avoiding toxicity of surgery, radiation, or combination immunotherapy.
- Provides a model for studying exceptional responders to checkpoint blockade.
Impact on Treatment Paradigm
- Establishes desmoplastic melanoma as one of the most responsive cancers to PD-1 inhibition.
- Highlights pembrolizumab as a low-toxicity, durable, and paradigm-shifting therapy for patients who previously had no effective options.
- Informs broader immunotherapy strategies for cancers with high mutational burden and immune visibility.
Pembrolizumab achieves unprecedented long-term control in advanced desmoplastic melanoma, positioning anti-PD-1 therapy as the new standard of care for this rare but aggressive disease.
Journal article: Kendra, K.L. et al, 2025. Anti-PD-1 therapy in unresectable desmoplastic melanoma: the phase 2 SWOG S1512 trial, Nature Medicine.
Summary by Stefan Botha










