Researchers looked at mechanisms in the skin that might lessen damage from UV radiation or infections, which can also produce uncomfortable symptoms like sunburn, in a new report that looked at inflammatory reactions in the skin. Now, a molecular regulator that incorporates these stress signals has been found by a recent research (Figure 1).
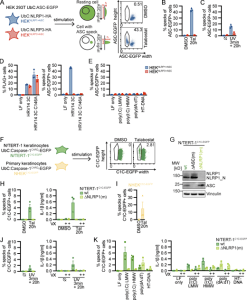
Figure 1: Reporter cell lines recapitulate NLRP1 inflammasome assembly. (A) Scheme of generated HEK 293T reporter cell lines and detection of ASC specks by flow cytometry. (B–E) HEKNLRP1+ASC or HEKNLRP3+ASC cells were treated with 30 µM Tal (B) or UV for 3 min (C), or were transfected with expression vectors for FLAG-tagged HRV 14 protease 3C (D), or 1 µg/ml of the indicated nucleic acid species (E). 20 h after treatment, ASC-EGFP–positive cells were analyzed by flow cytometry and the fraction of cells with ASC specks was determined with the gating strategy described in A. In D, cells were additionally stained for FLAG and ASC specks were only quantified in FLAG-positive cells for all samples transfected with plasmids, i.e., not in LF only controls. (F) Scheme of generated N/TERT-1 reporter cell lines and detection of C1C specks by flow cytometry. (G) N/TERT-1C1C-EGFP cells and monoclonal ASC and NLRP1 knockout derivatives were analyzed by immunoblot with the indicated antibodies. (H–K) N/TERT-1C1C-EGFP cells and their monoclonal NLRP1 knockout derivatives (H, J, and K) or NHEKC1C-EGFP (I) were treated with the indicated stimuli for 20 h as described above. For flow cytometry experiments, cells were additionally treated with 100 µM VX. To quantify inflammasome assembly, C1C-EGFP–positive cells were analyzed by flow cytometry. The fraction of cells with C1C-EGFP specks was determined with the gating strategy described in F. IL-1β from the supernatants of cells stimulated in the absence or presence of 100 μM VX was quantified by HTRF (H, J, and K). Data represents average values (with individual data points) from three independent experiments ± SEM. UbC indicates ubiquitin C (promoter). MW, molecular weight.
The skin acts as the body’s biggest organ and a main barrier against viruses and external stimuli. As everyone who has ever suffered a sunburn knows, once this barrier is breached, painful inflammation can build. But until now, it remained unclear exactly how this is started.
UV light can be very harmful. When it strikes the skin, it may harm crucial biological components, leading frequently to inflammation. They were able to demonstrate in this research that these inflammatory reactions may be brought on by a recognized cellular stress signaling system.
Ribosomes typically put together proteins in accordance with the instructions included in the genetic code. They raise the alert when it is compromised by UV damage by inducing the so-called ribotoxic stress response. This initiates a signaling cascade that activates the p38 enzyme. In their study, scientists demonstrated that p38 triggers NLRP1, a crucial switch for inflammation in the skin, in a unique way by molecularly altering it. Assembling inflammasomes from various molecular building blocks is then started as a result (READ MORE).
It’s interesting to note that p38 is not just triggered by too much sun exposure. They were able to demonstrate that viruses carried by mosquitoes may also activate NLRP1 through p38.
Investigation of p38 may hold the answer to many researcher questions surrounding the immune environment/reactions of/in the skin.
Journal article: Jenster, L.M., et al., 2022. P38 kinases mediate NLRP1 inflammasome activation after ribotoxic stress response and virus infection. Journal of Experimental.
Summary by Stefan Botha










