Hrpes simplex virus 1 (HSV-1) infects over 3.8 billion people worldwide, according to the World Health Organization. HSV-1 is also linked to viral encephalitis (brain inflammation) and has been implicated in Alzheimer’s disease.
Researchers have uncovered a surprising mechanism behind (HSV-1 reactivation, providing a potential new target to prevent cold sores and genital herpes outbreaks (Figure 1). The discovery reveals that the virus actively initiates its own reawakening by triggering the body’s antiviral defences, a strategy that seems counterintuitive but benefits the virus.
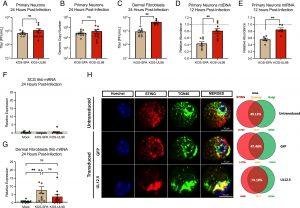
Figure 1: UL12.5 does not affect lytic replication in peripheral neurons. (A and B) Primary neurons isolated from the SCG of newborn mice were infected at an MOI of 10 PFU/cell. Viral titer (A) and viral genome copy number quantified by qPCR (B) were measured at 24 h postinfection with either KOS-SPA (WT) or KOS-UL98 (UL12.5 null). (C) Dermal fibroblasts isolated from newborn mice were infected at an MOI of 3 PFU/mL and viral titer quantified at 24 h postinfection. (D and E) Relative abundance of (D) mtDLoop1 mtDNA and (E) mtCOX2 mtRNA transcripts measured by (RT-) qPCR 12 h after infection with KOS-SPA or KOS-UL98. (F and G) Relative Ifnb mRNA expression 24 h after infection of (F) primary sympathetic neurons or (G) dermal fibroblasts (H) Representative immunofluorescence images of STING/TGN46 at 3-d posttransduction with either GFP or UL12.5 expressing lentiviral vector. UL12.5-mediated STING activation is indicated by its translocation and accumulation in the Golgi compartment, with 74.18% of the STING protein overlapping with Golgi marker TGN46. (D and E) The dotted line represents the abundance of mtDNA/RNA in uninfected neurons. (F and G) The dotted line represents background basal levels of viral transcripts in uninfected neurons. All in (A–G) data are plotted as biological replicates from at least three independent experiments, shown as mean ± SEM. Statistical comparisons were made using a t test or one-way ANOVA with Tukey’s multiple comparison test. *P < 0.05, **P < 0.01.
HSV-1, responsible for cold sores and genital herpes, can remain dormant in nerve cells for long periods. While stress, infections, and even sunburns are known to trigger reactivation, the team found that the virus itself plays a role in waking up from dormancy.
- The virus produces a protein called UL12.5, which stimulates the body’s immune response in infected neurons.
- Instead of harming the virus, this host defence mechanism is exploited, allowing HSV-1 to reactivate and spread.
- This discovery challenges conventional thinking, as triggering the immune system would typically be seen as a disadvantage for a virus.
The researchers also found that HSV-2, which primarily causes genital herpes, uses the same UL12.5 protein for reactivation, suggesting that targeting this viral protein could lead to treatments for both forms of herpes.
One striking finding was that UL12.5 is not needed when the body is already fighting another infection. The researchers believe that other pathogens trigger immune “sensing pathways” in neurons, which may be enough to activate herpes replication. This explains why common infections often provoke herpes outbreaks.
This discovery revolutionizes the understanding of herpes virus reactivation, identifying UL12.5 as a key target for future treatments. By blocking the virus’s ability to reawaken, scientists could potentially develop new drugs to prevent cold sores, genital herpes, and other herpes-related complications.
Journal article: Krakowiak, P.A., et al., 2025. Co-option of mitochondrial nucleic acid–sensing pathways by HSV-1 UL12.5 for reactivation from latent infection. Proceedings of the National Academy of Sciences.
Summary by Stefan Botha










