HIV-1 is initially released from infected cells in an immature and non-infectious form. The virus structure is primarily composed of 2,000 copies of a rod-shaped protein called Gag, which must undergo a major transformation before the virus becomes capable of infecting new cells.
- HIV maturation is triggered by the viral enzyme HIV-1 protease, which cuts Gag into six smaller proteins, including the capsid and matrix proteins.
- This proteolytic cleavage causes a dramatic rearrangement of the virus’s structural components, particularly the capsid, which encloses the viral genome.
While capsid transformations have been extensively studied, less was known about the changes in the viral matrix—the outer protein layer just beneath the virus’s lipid membrane.
Scientists have identified a key mechanism in the life cycle of HIV-1, revealing how the virus transitions from an immature state into an infectious form (Figure 1). Their study highlights the previously unknown role of spacer peptide 2, a viral component crucial for HIV maturation and infectivity.
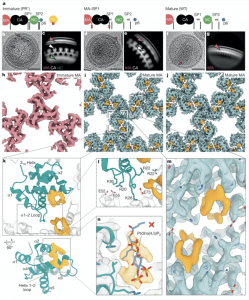
Figure 1: High-resolution reconstructions of MA in immature, mature and MA–SP1 particles. a, A schematic representation of the Gag cleavage state for each sample. b, Representative image of immature (PR−) HIV-1 virion from cryo-EM data (Extended Data Table 1). Arrowheads highlight specific Gag domain layers (red, MA; white, immature CA; green, NC). c, Side-view 2D class of the Gag layer. Arrowheads as in b. d, Representative MA–SP1 HIV-1 virion (Extended Data Table 1). e, Side-view 2D class of the Gag layer. As expected, MA and immature CA layers are seen, but NC has been cleaved. Arrowheads as in b. f, Representative mature (WT) HIV-1 virion (Extended Data Table 1). g, Side-view 2D class of the MA layer. Only the MA layer is visible because CA has been cleaved and undergone maturation. h–j, 3D cryo-EM reconstructions of the MA lattices, viewed from the membrane. As previously observed1, the MA lattice was found to be immature in immature (PR−) particles (h), and mature in MA–SP1 (i) and mature (WT) (j) particles. Additional ligand density is observed (orange) in both mature MA lattices, but was absent in the immature lattice. k, Top: top view of the model of the mature MA lattice, including the ligand density. α1, α-helix 1. One MA monomer is blue; surrounding monomers are white. Bottom: rotated view with helices labelled. l, Zoom in of the trimer–trimer interface in the atomic model of the mature MA lattice. The interface is mediated by electrostatic interactions between HBR residues and an electronegative patch along the intra-trimeric interface. m, Zoomed-in view of i showing ligand binding across the trimer–trimer interface with fitted atomic model (grey). n, Rigid fitting of the deposited model of MA bound to PtdIns(4,5)P2 (Protein Data Bank accession code 2H3Q)12 shows that the ligand density is not consistent with PtdIns(4,5)P2 bound as previously reported (the PtdIns(4,5)P2 atomic model is not accommodated within the orange EM density). Scale bars, 50 nm.
Using advanced cryo-electron microscopy, the research team produced detailed 3D structural models of HIV-1 virus particles. Their findings revealed a previously unknown function of spacer peptide 2 (SP2)—one of the six protein fragments generated from Gag cleavage.
Key Findings:
✔️ SP2 binds to the matrix protein after its release, triggering structural rearrangements that are essential for the virus’s transition to an infectious state.
✔️ This binding interaction accelerates the fusion of HIV-1 with target cells, facilitating viral spread.
✔️ The matrix has a pocket that accommodates SP2, stabilizing the mature virus structure—a feature that could serve as a potential drug target for future HIV treatments.
The mature form of the HIV matrix features a binding pocket where SP2 interacts with the matrix proteins. Initially, scientists assumed this pocket was occupied by a lipid from the viral membrane, but high-resolution imaging revealed that SP2 itself binds to this site.
Potential Drug Target:
- Since SP2 is critical for the virus’s infectivity, blocking its interaction with the matrix protein could disrupt HIV maturation, providing a new therapeutic strategy against the virus.
This discovery adds a critical piece to the puzzle of HIV maturation, identifying SP2 as a key regulator of the virus’s structural transformation. With further research, drugs that disrupt SP2 function could provide a novel approach to HIV treatment, potentially preventing the virus from becoming infectious.
Journal article: Stacey, J.C.V, et al, 2025. The conserved HIV-1 spacer peptide 2 triggers matrix lattice maturation, Nature.
Summary by Stefan Botha










