In a new study, researchers have made significant progress in our understanding of the intricate cellular process responsible for triggering a crucial inflammatory response in the body (Figure 1). Specifically, they have gained insights into the steps leading to the production of IL-1 beta, a potent inflammatory protein signal released during various inflammatory reactions.
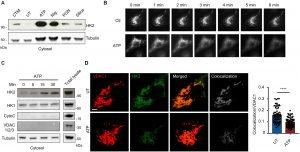
Figure 1: Common NLRP3 inflammasome activators trigger HK release from mitochondria. (A) Immunoblot of HK2 and tubulin in cytosolic fractions from mouse BMDMs primed with LPS for 3 hours and left untreated (UT) or activated with ATP (5 mM; 30 min), nigericin (Nig; 3 μM; 30 min), PGN (40 μg/ml; 6 hours), or silica particles (20 μg/ml; 6 hours). Clotrimazole (CTM) treatment was used as a positive control for HK release from mitochondria. (B) LPS-primed BMDMs expressing HK2-GFP were imaged before and after treatment with 5 mM ATP as indicated. (C) Immunoblot time course of HK2 and HK1 together with controls cytochrome c (CytoC), VDAC 1/2/3, and tubulin in cytosol fractions from BMDMs stimulated with ATP (5 mM) for the indicated periods. (D) STED images of LPS-primed BMDMs costained with antibodies to VDAC1 and HK2 and stimulated or not with ATP (5 mM; 1 hour). Scale bar, 3 μm. Colocalization was quantified in multiple cells (n = 59 to 65 cells across five independent experiments). Data are presented as means ± SEM. ****P < 0.0001 (unpaired t test).
Chronic inflammation, characterized by an immune system stuck in a persistent state of inflammation, can have detrimental effects on the body. It is associated with the development of serious conditions such as Type 2 diabetes and cardiovascular disease as it damages healthy cells.
In this study, scientists made discovered that the enzyme hexokinase also serves a secondary function related to inflammation. Specifically, it binds to a sugar molecule derived from the bacterial cell wall, leading to the activation of inflammasomes. Inflammasomes are receptors of the innate immune system that recognize microbial invaders and tissue damage.
The researchers further observed that hexokinase relocates from the mitochondria, the cellular organelles responsible for energy generation. This relocation initiates an immune response, as the release of hexokinase destabilizes the mitochondria and triggers an alert within the cell. This, in turn, causes the clustering of a channel known as VDAC in the mitochondrial membrane, which interacts with another protein called NLRP3 to initiate the assembly of inflammasomes. The assembled inflammasomes then facilitate the production of IL-1 beta, a key driver of inflammation. The investigation involved studying cells derived from laboratory mice to elucidate the steps involved in the IL-1 beta pathway.
This newfound knowledge opens exciting possibilities for targeted interventions. By targeting specific steps in this pathway, researchers may be able to modulate inflammation effectively, while also preserving the essential cellular energy balance. Such advancements in our understanding of the intricate interplay between inflammation and cellular processes bring us closer to developing innovative therapies for a range of inflammatory conditions, ultimately leading to improved health outcomes for patients.
Journal article: Sung Hoon Baik, et al., 2023. Hexokinase dissociation from mitochondria promotes oligomerization of VDAC that facilitates NLRP3 inflammasome assembly and activation. Science Immunology.
Summary by Stefan Botha










