High-grade serous ovarian cancer is the most lethal form of ovarian cancer. It is often diagnosed late, responds initially to treatment, but frequently relapses, making long-term survival rare. One major reason immunotherapy has failed in ovarian cancer is that cancer-associated fibroblasts (CAFs), non-cancerous cells that surround tumours, act as immune suppressors, shielding the tumour from attack.
Researchers have discovered how to block an enzyme called NNMT, which plays a central role in helping ovarian tumours evade the immune system (Figure 1). By inhibiting NNMT, the team not only reduced tumour growth in preclinical models of high-grade serous ovarian cancer but also restored immune activity, offering new hope for overcoming treatment resistance in this deadly cancer type.
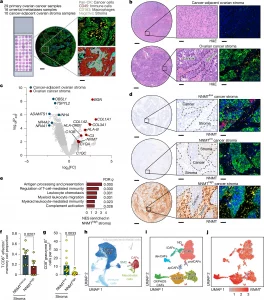
Figure 1: NNMT upregulation in CAFs is associated with decreased CD8+ T cell activation in HGSOC. a, Spatial transcriptomics analysis of a TMA, consisting of 55 samples from 30 patients with HGSOC. Top right, immunofluorescence staining of pan-CK (green), CD45 (red) and CD163 (yellow); nuclear staining is visible in blue. Bottom right, area of interest (AOI) selection; red, cancer cells; aqua, macrophages; magenta, other immune cells; grey, unstained stroma. Scale bars, 250 μm (left) and 50 μm (right). b, H&E staining of TMA cores from non-cancerous cancer-adjacent ovarian stroma and HGSOC stroma (left), with higher-magnification images identifying stroma and cancer cells (middle). Right, immunofluorescence staining of the corresponding core using the markers described in a. Scale bars, 250 μm (left) and 50 μm (middle and right). c, Differentially expressed genes (DEGs) from spatial transcriptomics data of cancer-adjacent ovarian stroma (n = 10) and HGSOC stroma (n = 27). Benjamini–Hochberg correction for false-discovery rate (FDR) was used. FC, fold change; Padj, adjusted P value. d, Immunohistochemistry staining for NNMT was performed on the TMA slide and stroma was scored as NNMTlow, NNMTint and NNMThigh (left and middle). Right, immunofluorescence staining of the corresponding core using the markers described in a. Scale bars, 250 μm (left) and 50 μm (middle and right). e, Upregulation of selected pathways in NNMThigh stroma (n = 24) compared with NNMTlow stroma (n = 16), identified by GSEA using the human GO Biological Pathway (GOBP) gene sets. Benjamini–Hochberg correction for false-discovery rate (FDR) was used. NES, normalized enrichment score. f, Immune deconvolution of spatial transcriptomics: the effector/memory CD8+ T cell proportion among CD45+CD163− immune cells in NNMTlow/int (n = 9) and NNMThigh stroma (n = 8). Statistical analysis was performed using two-tailed unpaired Student’s t-tests. g, TMA of an independent cohort of 27 HGSOC tissues were stained for NNMT (immunohistochemistry), and CD8 and granzyme B (immunofluorescence). The number of granzyme B+CD8+ T cells was counted and compared between NNMThigh (n = 11) and NNMTlow (n = 16) cores. Statistical analysis was performed using two-tailed unpaired Mann–Whitney U-tests. h, Uniform manifold approximation and projection (UMAP) of all cell types in omental metastases from scRNA-seq data of seven chemotherapy-naive patients with HGSOC. SMC, smooth muscle cells. i, Unsupervised Seurat clustering of CAFs identified eight subclusters: iCAFs, vCAFs, prolCAFs, apCAFs, OXPHOS CAFs, myCAFs, neuralCAFs and devCAFs. Two small clusters were classified as not defined (ND) owing to low cell counts (n < 100 cells). j, NNMT expression across all CAF subtypes (scaled mean expression). Data are mean ± s.e.m.
The enzyme nicotinamide N-methyl transferase (NNMT) is highly expressed in CAFs. Previous research showed that NNMT converts normal fibroblasts into tumour-promoting cells by rewiring their metabolism and gene regulation. In this new study, researchers uncovered how NNMT also enables immune evasion: it programs CAFs to release signals that convert immune cells into myeloid-derived suppressor cells (MDSCs) – potent suppressors of anti-tumour immunity.
The team screened over 150,000 compounds and identified a highly selective NNMT inhibitor. In animal models:
- The drug reduced tumour burden
- It reinvigorated the immune system
- And when combined with immune checkpoint inhibitors, it completely halted tumour growth
This study highlights a new approach: targeting the tumour microenvironment, not just the tumour itself.
By turning hostile support cells back into neutral, or even helpful allies, NNMT inhibition opens a promising path for precision immunotherapy in ovarian cancer and possibly beyond.
Journal article: Heide, J. et al, 2025. NNMT inhibition in cancer-associated fibroblasts restores antitumour immunity, Nature.
Summary by Stefan Botha










