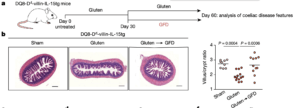
DQ8-Dd-villin-IL-15tg mice were maintained on a gluten-free diet (GFD; sham), fed gluten for 60 days (gluten), or fed gluten for 30 days and then reverted to a GFD (gluten→GFD) for 30 days. a, Experimental timeline. b, Left, haematoxylin and eosin (H&E) staining of paraffin-embedded ileum sections. Scale bars, 200 μm. Right, graph depicts the ratio of the morphometric assessment of villus height to crypt depth (GFD, n = 9; gluten, n = 13; gluten→GFD, n = 11 mice). c, Serum levels of anti-DGP IgG antibodies were measured by ELISA. Serum was collected sequentially in the same mice (n = 12) before gluten feeding (untreated), 30 days after gluten feeding (gluten d30), and 30 days after reversion to a GFD (GFD d60). (Source: Abadie et al., 2020)
Coeliac disease is an inflammatory autoimmune disease of the small intestine caused by anti-gluten innate and adaptive immunity. It is associated with over-expression of IL-15, presence of IgG and IgA antibodies against peptides and enzymes such as transglutaminase-2 (TG2) and cytolytic activity in the small intestines. Up-regulation of IL-15, predominantly from intestinal epithelial cells (IECs) is crucial for induction of cytotoxic T cells during coeliac disease. However, when researchers attempted to develop murine models of coeliac disease by up-regulating IL-15 secretion by either the lamina propria or IECs. They were unable to recapitulate pathophysiological and immunological features associated with coeliac disease. Additionally, neither model was able to induce villous atrophy, a key pathology associated with coeliac disease . Based on these results, Abadie et al., hypothesised that induction of coeliac disease required overexpression of IL-15 by both the lamina propria and IECs.
Abadie et al., developed transgenic DQ8-Dd-villin-IL-15tg mice, which express HLA-DQ8 [HLA associated with genetic predisposition to coeliac disease), as well as over-express IL-15 by both the lamina propria and IECs. These transgenic mice developed immunopathologies associated with coeliac disease, including villous atrophy after a gluten-rich diet. Using this model they demonstrated that “[The] adaptive TH1 immune response promoted by HLA-DQ8 and IL-15 in the lamina propria, synergises with IL-15 in the epithelium to further promote the expansion of cytolytic lymphocytes and their degranulation, leading to CD8+ T cell-dependent killing of epithelial cells and villous atrophy.”
These results represent the first murine model of coeliac disease and pave the way for pre-clinical research on potential therapeutic strategies as well as an animal model to enable improved understanding of coeliac disease immuno-pathology.
Abadie et al., 2020. IL-15, gluten and HLA-DQ8 drive tissue destruction in coeliac disease. Nature
Article by Cheleka AM Mpande











