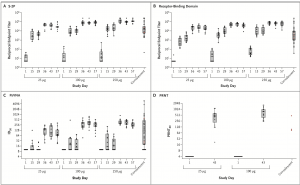
SARS-CoV-2 Antibody and Neutralization Responses. Shown are geometric mean reciprocal end-point enzyme-linked immunosorbent assay (ELISA) IgG titres to S-2P (Panel A) and receptor-binding domain (Panel B), PsVNA ID50 responses (Panel C), and live virus PRNT80 responses (Panel D). In Panel A and Panel B, boxes and horizontal bars denote interquartile range (IQR) and median area under the curve (AUC), respectively. Whisker endpoints are equal to the maxi- mum and minimum values below or above the median ±1.5 times the IQR. The convalescent serum panel includes specimens from 41 participants; red dots indicate the 3 specimens that were also tested in the PRNT assay. The other 38 specimens were used to calculate summary statistics for the box plot in the convalescent serum panel. In Panel C, boxes and horizontal bars denote IQR and median ID50, respectively. Whisker end points are equal to the maxi- mum and minimum values below or above the median ±1.5 times the IQR. In the convalescent serum panel, red dots indicate the 3 specimens that were also tested in the PRNT assay. The other 38 specimens were used to calculate summary statistics for the box plot in the convalescent panel. In Panel D, boxes and horizontal bars denote IQR and median PRNT80, respectively. Whisker end points are equal to the maximum and minimum values below or above the median ±1.5 times the IQR. The three convalescent serum specimens were also tested in ELISA and PsVNA assays. Because of the time-intensive nature of the PRNT assay, for this preliminary report, PRNT results were available only for the 25-μg and 100-μg dose groups. (Source: Jackson et al., NEJM)
Safety and immunological results from the phase 1 COVID-19 trial testing, the candidate vaccine mRNA-1273, were published in NEJM on 14th July. mRNA-1273 is a lipid nanoparticle–encapsulated, nucleoside-modified messenger RNA (mRNA)– based vaccine that encodes the SARS-CoV-2 spike (S) glycoprotein stabilized in its prefusion conformation. The phase 1 clinical trial, which aimed to determine the safety profile of mRNA-1273 began in March 2020 (66 days after the genomic sequence of the virus was posted). Overall vaccination with mRNA-1273 did not result in severe side-effects, however, the frequency of side effects correlated with vaccine antigen concentration.
Researchers reported rapid seroconversion, within 15 days of the first vaccination dose in all participants. However, only vaccination with highest doses (100μg and 200μg) resulted in induction of vaccine-induced spike-protein specific antibodies at similar magnitudes as those observed in convalescent samples. Additionally, they showed that induction of robust neutralising antibody titres, with neutralisation capacity as those observed in convalescent samples, required two vaccination doses. Lastly, they showed that mRNA-1273 induced robust spike-protein-specific CD4 T cells responses, which predominantly produced Th1 associated cytokine responses, but very low levels of vaccine-specific CD8 T cells responses.
In summary, results from the phase 1 mRNA-1273 vaccine trial, though preliminary, clearly indicate that the mRNA-1273 vaccine is safe and immunogenic. Warranting further investigation in a phase 2 trial, which has already began, NCT04405076.
Journal Article: Jackson et al., 2020. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. NEJM
Summary by Cheleka AM Mpande










