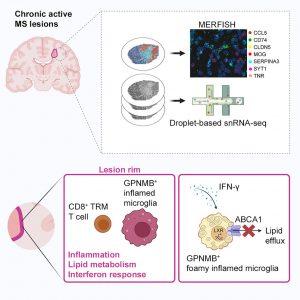
A new study maps how immune and brain cells interact to sustain long-term inflammation in multiple sclerosis (Figure 1).
Multiple sclerosis (MS) is often described as the immune system attacking the brain. But in progressive MS, the damage doesn’t come from sudden immune attacks, it smoulders slowly over years, driven by persistent inflammation deep within the brain. What keeps that inflammation alive has remained one of MS research’s biggest mysteries.
Now, scientists have uncovered an unexpected culprit: lipid-storing, dysfunctional microglia, the brain’s own immune cells.
Researchers created a high-resolution single-cell and spatial map of chronic active MS lesions, areas of slow, ongoing damage surrounded by inflamed immune cells. Using single-nucleus RNA sequencing (snRNA-seq) and MERFISH spatial transcriptomics, they traced how immune niches form within these lesions.
At the outer rims of MS lesions, the team discovered clusters of CD8⁺ T cells closely interacting with microglia, the brain’s resident immune cells. These microglia showed signs of interferon activation and altered lipid metabolism, suggesting they had become “foam-like” cells packed with fats.
To test whether this buildup of lipids affects inflammation, the researchers deleted two key cholesterol transport genes, ABCA1 and ABCG1, specifically in microglia in a mouse model of MS (experimental autoimmune encephalomyelitis, EAE). The result: microglia became overloaded with lipids, forming foam cells that worsened inflammation and tissue damage.
Conversely, when the team pharmacologically targeted sterol metabolism, it reduced lipid accumulation and protected against inflammatory demyelination, pointing to a possible therapeutic strategy.
By combining cutting-edge genomics with spatial imaging, this study provides the most detailed view yet of the cellular neighbourhoods that sustain chronic inflammation in MS. It highlights how lipid metabolism, long known to shape cardiovascular disease, also plays a central role in neuroinflammatory disorders.
The findings open new directions for MS therapy. Rather than focusing solely on immune suppression, treatments that reprogram microglial lipid handling might help break the cycle of inflammation that drives neurodegeneration.
Chronic MS lesions aren’t just scars; they’re metabolically active ecosystems. And at their centre are microglia gone awry, overloaded with lipids that fuel inflammation and prevent healing.
Journal article: Feng, R., et al. 2025. Single-cell spatial transcriptomic profiling defines a pathogenic inflammatory niche in chronic active multiple sclerosis lesions. Immunity.
Summary by Stefan Botha