 IUIS-ALACI-ACAAI Immuno-Colombia 2021 course took place remotely between 5th to 16th April. The theme of this meeting was “Mechanisms and Approaches to Immunotherapy for Cancer and Chronic Inflammatory Diseases”. This article highlights a talk by Professor Soraya Zorro which focused on Tumour Infiltrating Lymphocytes (TIL) therapy.
IUIS-ALACI-ACAAI Immuno-Colombia 2021 course took place remotely between 5th to 16th April. The theme of this meeting was “Mechanisms and Approaches to Immunotherapy for Cancer and Chronic Inflammatory Diseases”. This article highlights a talk by Professor Soraya Zorro which focused on Tumour Infiltrating Lymphocytes (TIL) therapy.
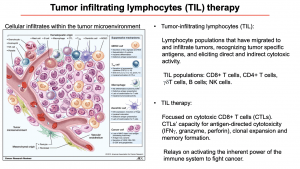 Professor Soraya Zorro began her lecture with an overview of TILs and TILs therapy. TILs are lymphocyte populations that migrate to and infiltrate the tumour. These lymphocytes recognise specific antigens, elicit direct and indirect cytotoxic activity. The main populations that can be found infiltrating in the tumour microenvironment are CD8+ T cells, CD4+ T cells, γδ T cells, B cells and NK cells. TIL therapy predominantly uses cytotoxic CD8+ T cells (CTLs) as agents of immunotherapy, due to their antigen-specific cytotoxicity, clonal expansion, and memory formation.
Professor Soraya Zorro began her lecture with an overview of TILs and TILs therapy. TILs are lymphocyte populations that migrate to and infiltrate the tumour. These lymphocytes recognise specific antigens, elicit direct and indirect cytotoxic activity. The main populations that can be found infiltrating in the tumour microenvironment are CD8+ T cells, CD4+ T cells, γδ T cells, B cells and NK cells. TIL therapy predominantly uses cytotoxic CD8+ T cells (CTLs) as agents of immunotherapy, due to their antigen-specific cytotoxicity, clonal expansion, and memory formation.
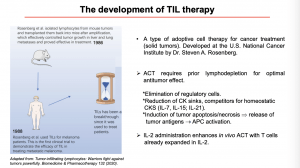 Next, she gave an overview of how Dr. Steven A. Rosenberg and colleagues developed the TIL therapy (Lin B. et al, 2020), using pre-clinical murine studies. During his studies lymphocytes were isolated from mouse tumours and transplanted back into mice after amplification, effectively controlling tumour growth in liver and lung metastases. In 1988, the technique was used to treat melanoma patients enrolled in clinical trials. During the clinical trial, TIL therapy was made more effective by including prior lymphodepletion for optimal anti-tumour effect and IL-2 administration to enhance in vivo activation of T cells.
Next, she gave an overview of how Dr. Steven A. Rosenberg and colleagues developed the TIL therapy (Lin B. et al, 2020), using pre-clinical murine studies. During his studies lymphocytes were isolated from mouse tumours and transplanted back into mice after amplification, effectively controlling tumour growth in liver and lung metastases. In 1988, the technique was used to treat melanoma patients enrolled in clinical trials. During the clinical trial, TIL therapy was made more effective by including prior lymphodepletion for optimal anti-tumour effect and IL-2 administration to enhance in vivo activation of T cells.
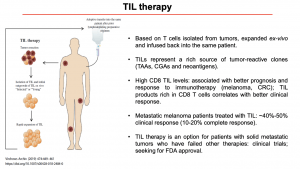 Prof Zorro then discussed immunotherapy is administered, specifically how anti-tumour T cells isolated from tumours are expanded ex-vivo and infused back into the same patient (Rohaan M. W., Wilgenhof S., Haanen J. B. A. G., 2018). TILs represent a rich source of tumour-reactive clones, and high levels of CD8 TILs have been shown to correlate with a better clinical response. Currently, TIL therapy is only accessible to patients who have had unsuccessful cancer therapies and agree to enrol in clinical trials to access the cutting edge therapy. Another aspect that was raised during the lecture was the fact that TIL Therapy may not be an option for every type of cancer, to be a good candidate it is necessary to seek for tumours with a high mutational burden (Martincorena I. and Campbell P.J., 2015), and have a high proportion of TILs, and a diverse and reactive T cell repertoire.
Prof Zorro then discussed immunotherapy is administered, specifically how anti-tumour T cells isolated from tumours are expanded ex-vivo and infused back into the same patient (Rohaan M. W., Wilgenhof S., Haanen J. B. A. G., 2018). TILs represent a rich source of tumour-reactive clones, and high levels of CD8 TILs have been shown to correlate with a better clinical response. Currently, TIL therapy is only accessible to patients who have had unsuccessful cancer therapies and agree to enrol in clinical trials to access the cutting edge therapy. Another aspect that was raised during the lecture was the fact that TIL Therapy may not be an option for every type of cancer, to be a good candidate it is necessary to seek for tumours with a high mutational burden (Martincorena I. and Campbell P.J., 2015), and have a high proportion of TILs, and a diverse and reactive T cell repertoire.
In the next article, we shall discuss how cells for TIL therapy are produced, considerations for TIL therapy and utility of TIL therapy with other cancer immunotherapies.
- Interested in learning more about TILs: read advanced pre-course material on Tumor-infiltrating Lymphocytes (TIL)
- Full lecture recording available at IUIS-ALACI-ACAAI Immuno-Colombia 2021 Lecture week 2
Summary by Carla Sanzochi Fogolin










