IUIS-ALACI-ACAAI Immuno-Colombia 2021 course took place remotely between 5th to 16th April. The theme of this meeting was “Mechanisms and Approaches to Immunotherapy for Cancer and Chronic Inflammatory Diseases”. Today, we highlight a talk by Professor Elizabeth Quackenbush (Immune disease institute, Boston) entitled “Checkpoint Blockade-based Therapies”
IUIS-ALACI-ACAAI Immuno-Colombia 2021 course took place remotely between 5th to 16th April. The theme of this meeting was “Mechanisms and Approaches to Immunotherapy for Cancer and Chronic Inflammatory Diseases”. Today, we highlight a talk by Professor Elizabeth Quackenbush (Immune disease institute, Boston) entitled “Checkpoint Blockade-based Therapies”
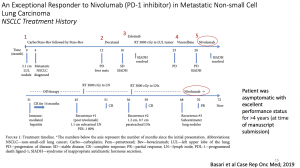 In this summary, we shall highlight a case study on Checkpoint Blockade-based Therapies presented by Professor Queckenbush during the lecture. This case highlights an exceptional responder to immune checkpoint inhibitors in metastatic non-small-cell lung cancer (NSCLC). A 73-year-old man with active tobacco dependence achieved complete response with nivolumab for metastatic NSCLC after four prior lines of chemotherapy. Nivolumab was then discontinued after 10 cycles due to immune-mediated hepatitis that resolved with steroids. He remained in complete remission for 14 months while off therapy. However, his tumour recurred twice locally in the mediastinum and was treated both times with radiotherapy. Due to recurrence in both lungs one year later, he was rechallenged with nivolumab and achieved partial response after two months of therapy. He continues to do well five and a half years since his initial diagnosis of de novo metastatic NSCLC. This case was unique in several aspects. First, it is a real-world scenario of an exceptional responder to immunotherapy outside of a clinical trial. Second, he had been diagnosed with metastatic lung cancer for more than 5.5 years and continues to do well with limited disease burden following eight lines of therapy, including radiotherapy. Third, he exhibited many of the predictive markers mentioned including high PD-L-1 expression (80%), smoking history, prior radiotherapy and bevacizumab use, and development of immune-mediated hepatitis requiring nivolumab to be withheld. Interestingly, unlike what is reported in the literature on poor responses to checkpoint inhibitors in liver metastasis, this patient had a complete response to nivolumab and disease never recurred in his liver despite having a relatively large liver metastasis, possibly due to the activation of the immune system that resulted in hepatitis. Unfortunately, not all patients will experience favourable responses to treatment with checkpoint inhibitors and may even suffer from severe side effects. Therefore, prognostic and predictive markers, beyond programmed death-ligand 1 (PD-L1) expression status are of utmost importance for decision making in palliative treatment. Prof. Quackenbush then focused on clinical (male gender, administration of steroids and antibiotics, BMI, liver and pleural metastasis), laboratory (LDH, CRP, blood count) and genetic markers (High Tumor mutational Burden, Circulating Tumor DNA, high PDL1), most of them easy to obtain in the daily clinical practice.
In this summary, we shall highlight a case study on Checkpoint Blockade-based Therapies presented by Professor Queckenbush during the lecture. This case highlights an exceptional responder to immune checkpoint inhibitors in metastatic non-small-cell lung cancer (NSCLC). A 73-year-old man with active tobacco dependence achieved complete response with nivolumab for metastatic NSCLC after four prior lines of chemotherapy. Nivolumab was then discontinued after 10 cycles due to immune-mediated hepatitis that resolved with steroids. He remained in complete remission for 14 months while off therapy. However, his tumour recurred twice locally in the mediastinum and was treated both times with radiotherapy. Due to recurrence in both lungs one year later, he was rechallenged with nivolumab and achieved partial response after two months of therapy. He continues to do well five and a half years since his initial diagnosis of de novo metastatic NSCLC. This case was unique in several aspects. First, it is a real-world scenario of an exceptional responder to immunotherapy outside of a clinical trial. Second, he had been diagnosed with metastatic lung cancer for more than 5.5 years and continues to do well with limited disease burden following eight lines of therapy, including radiotherapy. Third, he exhibited many of the predictive markers mentioned including high PD-L-1 expression (80%), smoking history, prior radiotherapy and bevacizumab use, and development of immune-mediated hepatitis requiring nivolumab to be withheld. Interestingly, unlike what is reported in the literature on poor responses to checkpoint inhibitors in liver metastasis, this patient had a complete response to nivolumab and disease never recurred in his liver despite having a relatively large liver metastasis, possibly due to the activation of the immune system that resulted in hepatitis. Unfortunately, not all patients will experience favourable responses to treatment with checkpoint inhibitors and may even suffer from severe side effects. Therefore, prognostic and predictive markers, beyond programmed death-ligand 1 (PD-L1) expression status are of utmost importance for decision making in palliative treatment. Prof. Quackenbush then focused on clinical (male gender, administration of steroids and antibiotics, BMI, liver and pleural metastasis), laboratory (LDH, CRP, blood count) and genetic markers (High Tumor mutational Burden, Circulating Tumor DNA, high PDL1), most of them easy to obtain in the daily clinical practice.
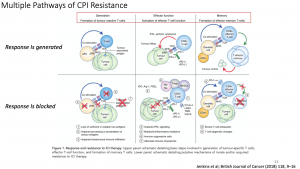
Unfortunately, not all patients will experience favourable responses to treatment with checkpoint inhibitors and may even suffer from severe side effects. Therefore, prognostic and predictive markers, beyond programmed death-ligand 1 (PD-L1) expression status are of utmost importance for decision making in palliative treatment. Prof. Quackenbush then focused on clinical (male gender, administration of steroids and antibiotics, BMI, liver and pleural metastasis), laboratory (LDH, CRP, blood count) and genetic markers (High Tumor mutational Burden, Circulating Tumor DNA, high PDL1), most of them easy to obtain in the daily clinical practice. Analysis of clinical trial data can identify three broad populations of patients, (i) those that respond initially and continue to respond (responders), (ii) those that fail to respond (innate (primary) resistance) and (iii) patients who initially respond but eventually develop disease progression (acquired (secondary) resistance). Challenges remain in defining responders and non-responders especially given the heterogeneity in patterns of response observed with immune checkpoint inhibitors. However, the initial treatment response appears to be a binary event, with most patients being non-responders to single checkpoint inhibitor therapy and further progressing to diseases. In addition, late relapses are now emerging with longer follow-up of clinical trial participants, suggesting a high prevalence of acquired resistance. As robust biomarkers to predict clinical response or resistance remain elusive, the mechanisms underlying innate and acquired resistance are largely inferred from pre-clinical studies and correlative clinical data. Improved understanding of molecular and immunologic mechanisms of immune checkpoint inhibitors responses (and resistance) may identify novel predictive or prognostic biomarkers, as well as ultimately guide optimal combination/sequencing of ICI therapy in the clinic.
- Interested in learning more about checkpoint inhibitors: read advanced pre-course material on Check-Points Blockade Based Therapies
- Full lecture recording available at IUIS-ALACI-ACAAI Immuno-Colombia 2021 Lecture week 1
Summary by Ichrak Bannour