In a new study published in PLOS Pathogens, researchers have demonstrated how specific features on the surface of HIV-infected cells play a key role in immune system evasion (Immunity to HIV). Adeniji, et al., have reported that HIV-infected cells are using a type of sugar molecule, sialic acid, to evade natural killer immune surveillance. In addition, the study has revealed an mechanism that inhibits HIV-infected cells whilst leaving healthy cells unaffected. The authors have identified a novel mechanism, glyco-immune checkpoint interaction, which allows HIV-infected cells to evade immune surveillance. They further developed a novel approach that selectively targets these interactions on the surface of these infected cells in order to inhibit or break these interactions.
Sialic acid is expressed on the surface of HIV-infected cells. These sugars bind to the surface of natural killer (NK) cells. Siglec-9 is an MHC-independent inhibitory receptor expressed on a subset of natural killer cells. Siglec-9 restrains NK cytotoxicity by binding to sialoglycans (sialic acid-containing glycans) on target cells. Siglec-9 functions within their novel identified mechanism as a “glyco-immune negative checkpoint.”
Through the use of in vivo phenotypic analyses, Adeniji identified that Siglec-9+ CD56dim NK cells, during HIV infection, exhibit an activated phenotype with higher expression of activating receptors and markers (NKp30, CD38, CD16, DNAM-1, perforin) and lower expression of the inhibitory receptor NKG2A, compared to Siglec-9– CD56dim NK cells (Figure 1).
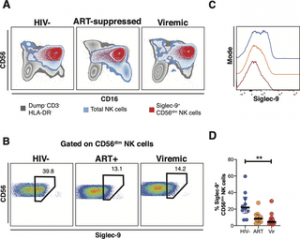
Figure 1: Expression of Siglec-9+ CD56dim NK cells during HIV infection. (A) Overlay plots showing the distribution of Siglec-9+ CD56dim NK cells (red) compared with total NK cells (blue) and non-T cell lymphocytes (grey). (B-C) Representative plots showing the frequency (B) and expression (MFI overlay) (C) of Siglec-9 in total CD56dim NK cells in HIV- (blue line), HIV+ ART+ (orange line), and HIV+ viremic (red line) individuals. (D) Decreased frequency of Siglec-9+ CD56dim NK cells during HIV infection compared to HIV- controls. Lines in graphs indicate the median of the group. ** p<0.01. Mann-Whitney rank test was used to compare between groups. n = 10 HIV-negative controls, 11 HIV+ viremic, and 10 HIV+ on suppressive ART (Adeniji, et al., 2021).
The levels of Siglec-9+ CD56dim NK cells inversely correlate with viral load during viremic infection and CD4+ T cell-associated HIV DNA during suppressed infection (Figure 2). It is also important to note that Siglec-9+ NK cells exhibit higher cytotoxicity towards HIV-infected cells compared to Siglec-9– NK cells.
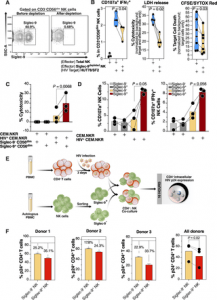
Figure 2: iglec-9+ CD56dim NK cells exhibit higher cytotoxicity towards HIV+ cells compared to Siglec-9- CD56dim NK cells. (A) A representative example of depletion of Siglec-9+ NK cells. (B) Siglec-9depleted NK cells exhibit lower cytotoxicity towards HIV-infected HUT78/SF2 targets compared to total NK cells. Cytotoxicity was assessed using NK degranulation, left panel (n = 3 donors; E:T = 4:1), LDH release, middle panel (n = 4 donors; E:T = 10:1), and CFSE/SYTOX Red assay, right panel (n = 6 donors; E:T = 10:1). NK degranulation measured as CD107a+ IFNγ+. Assays from each donor were performed in 2–4 replicates, and the average of these replicates per donor was used for statistical analyses. Statistical analyses were performed using paired t-tests. (C-D) FACS sorted Siglec-9+ CD56dim NK cells exhibit higher cytotoxicity towards HIV+ CEM.NKR targets compared to Siglec-9- CD56dim NK cells. (C) Cytotoxicity was assessed using LDH release assay (n = 3 donors, E:T = 10:1). (D) Analysis of NK degranulation (n = 3 donors; E:T = 4:1) was made on total NK cells gated on Siglec-9+ or Siglec-9- CD56dim NK cell subsets. Siglec-9+ = Siglec-9+ CD56dim NK cells and Siglec-9- = Siglec-9- CD56dim NK cells. Statistical analyses were performed using paired t-tests. (E) A schematic representation of the workflow to evaluate the cytotoxic potential of Siglec-9+ and Siglec-9- CD56dim NK cells against autologous HIV-infected CD4+ T cells. CD4+ T cells were isolated from fresh PBMC and exposed to HIV-1 IIIB for 72 h. On the third day, effector NK cells were isolated from PBMC of the same donor, FACS sorted, and co-cultured with autologous HIV-infected CD4+ T cells for 16 h. Following overnight incubation, the mixtures were stained for live/dead viability, CD3, and intracellular p24. (F) Data from the experimental design shown in (D). Dashed lines denote the percentage of p24+ cells in control HIV-infected CD4+ T cells cultured without effector cells. Percentages are percent reduction from dashed line. Assay from each donor was performed in triplicate (E:T = 10:1; n = 3 donors). Statistical analysis was performed using paired t-test (Adeniji, et al., 2021).
In short, the team discovered that the infected cells can take advantage of this inhibitory connection to evade immune surveillance. They then investigated whether they could interfere with this interaction/connection. The researchers identified and developed a sialidase conjugate linked to HIV antibodies (Figure 3) which was an effective approach towards manipulating the interaction. This antibody-sialidase conjugate only targeted sialic acid on HIV cells. This allowed immune cells to target HIV-infected cells while leaving healthy unaffected.
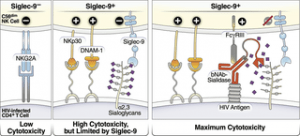
Figure 3: Model of how HIV bNAb-Sialidase conjugates may increase the cytotoxicity of Siglec-9+ NK cells against HIV-infected cells. Left two panels: The Siglec-9+ CD56dim NK subset has high cytolytic activity, possibly due to elevated expression of several NK activating receptors and reduced expression of the inhibitory NKG2A, compared to Siglec-9- CD56dim NK cells. However, Siglec-9 itself is an inhibitory receptor whose signaling restrains the cytolytic ability of these otherwise highly cytotoxic Siglec-9+ CD56dim NK cells by binding to Sialic acid attached to protein or lipid backbones on the surface of target cells. Right panel: Siglec/Sialic acid interactions are being pursued as an approach to enhance NK cell cytotoxicity against cancer using antibodies conjugated to Sialidase. We developed a similar proof-of-concept approach–conjugating Sialidase to HIV bNAbs–that could be used in conjunction with strategies that reactivate HIV latently-infected cells to enhance NK cells’ capacity to clear HIV+ cells (Adeniji, et al., 2021).
In their own words:
“…our study is the first to describe Siglec-9+ CD56dim NK cells in vivo as an NK subpopulation that can be exploited by HIV-infected cells to evade immunosurveillance. Our study is also the first to describe that this population is highly cytotoxic but is being restrained by the inhibitory marker (Siglec-9) they express (analogous to PD1 expression in highly activated CD8+ T cells). Finally, we developed a proof-of-concept approach (bNAb-sialidase conjugates) to selectively disrupt Siglec-9/sialoglycan interactions between NK cells and HIV-infected cells. This approach represents a promising, novel immunotherapeutic tool to be used in the future (in concert with latency-reversal agents or during ART-cessation) to clear reactivated HIV latently-infected cells.”
Journal Article: Adeniji, et al., 2021. Siglec-9 Defines and Restrains a Natural Killer Subpopulation Highly Cytotoxic to HIV-infected Cells. PLOS pathogens.
Summary by Stefan Botha










