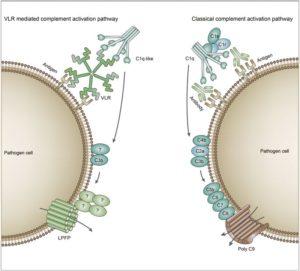
Comparison of the complement activation pathway of jawed and jawless vertebrates. In jawed vertebrates, the classical activation pathway of the complement system is triggered by antigen-antibody complex. The binding of complement C1 to the Fc region of the antibody initiates the complement activation pathway, and C4, C2 and C3 are then successively cleaved and activated. Finally, a subunit composition of C5b-C6-C7-C8-polyC9 forms the MAC that initiates the lysis of the target cells. In jawless vertebrates, the VLR-mediated complement pathway is triggered by antigen-VLR complex and C1q molecules. During this complement activation process, C3 is cleaved and activated. Finally, LPFP is recruited to form a pore complex on the membrane of target cells, resulting in the target cell lysis. Together, the two parallel complement pathways achieve similar cytotoxicity. (Source: Wu et al., 2017. Cell Discovery)
The complement system is a part of the immune system that forms the membrane attack complex (MAC). The classical complement pathway is initiated by antigen-antibody (Ab) complexes that bind to C1 in jawed vertebrates; Abs binds an antigen via its variable region while the Fc region binds C1q, leading to the cleavage and activation of C4, C2, and C3. Further downstream, C5b, C6, C7, C8, and C9 form the MAC. The MAC forms pores on target cells, disrupting the cell membrane and causing cell death. The lamprey, a jawless vertebrate, does not produce the traditional IgG or IgM Abs that initiate the antigen-Ab complex. Instead, lampreys have an alternate immune system that uses variable lymphocyte receptors (VLRs). These VLRs can control complement-dependent cytotoxicity independent of Abs. However, the final pieces of complement that form the MAC, C6 through C9, have not been observed in lampreys. In this study, the authors tried to find the molecules responsible for cell lysis in the terminal steps of VLR mediated cytotoxicity.
The researchers first confirmed that VLRs utilized a novel pore forming complex by studying the membrane proteins of E. coli, HeLa cells, and rabbit red blood cells before and after lamprey antisera treatment. With an inner diameter of 7-nm and an outer diameter of 17-nm, these VLR mediated pores had a different shape and size compared to other known pore forming complexes.
SDS page analysis and mass spectrometry of treated and untreated E. Coli membrane proteins showed that a previously uncharacterized natterin family protein was implicated in VLR mediated pore formation. This protein was named as lamprey pore-forming protein (LPFP). LPFP’s importance in cytotoxicity was confirmed when researcher were able to inhibit complement cytotoxicity after neutralizing LPFPs with specific Abs.
Lampreys possess an alternate immune system that employs VLR recombination instead of VDJ recombination as seen in jawed vertebrates. Previous studies showed that lamprey pore formation is initiated by antigen-VLR complexes and C1q. This study showed that LPFP forms the pore in the final step of VLR mediated cytotoxicity. Further VLR studies should be done to explore VLR’s therapeutic potential in cases where classical VDJ mediated therapies are ineffective.
Journal Article: Wu et al., 2017. A pore-forming protein implements VLR-activated complement cytotoxicity in lamprey. Cell Discovery
Article by Maxwell Chan











