New research reveals how oxygen deprivation leaves a lasting genetic imprint on the body’s infection-fighting cells (Figure 1).
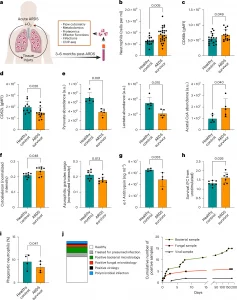
Figure 1: ARDS induces long-term alterations of neutrophil phenotypes and functions. a–i, Phenotypic and functional analysis of circulating neutrophils collected from healthy controls and survivors of ARDS 3–6 months post-hospital admission. a, Schematic representation of the analyses performed. Human silhouette adapted from Wikipedia commons (https://commons.wikimedia.org/wiki/File:Man_shadow_-_upper.png); lungs, neutrophils and blood tube adapted from Servier license under CC-BY 3.0 Unported. b, Circulating neutrophil counts obtained by flow cytometry (n = 19 healthy control and n = 26 ARDS survivor). c,d, Surface expression of the neutrophil activation markers CD66b and CD62L measured by flow cytometry (n = 15 healthy control and n = 14 ARDS survivor (c), n = 10 healthy control and n = 13 ARDS survivor (d)). e, Metabolite abundance of pyruvate, lactate and acetyl-CoA obtained by LC–MS analysis (n = 5 for both experimental groups). f, Proteomic analysis by LC–MS showing abundance of cytoskeletal (GO:0005856) and azurophilic granule cargo proteins (GO:0035578) (n = 8 for both experimental groups). g, Ex vivo quantification of α-1-antitrypsin from neutrophil culture supernatants performed by ELISA (n = 4 for both experimental groups, two technical replicates per sample). h, Ex vivo neutrophil survival in response to LPS stimulation after 20 h of culture evaluated by microscopy analysis (n = 7 for both experimental groups, two technical replicates per sample). i, Phagocytic capacity of opsonized S. aureus SH1000 measured by flow cytometry as a percentage of the total neutrophil population analyzed (n = 4 for both experimental groups, two technical replicates per sample). j, Infections recorded in ARDS survivors, with their infection etiologies expressed as proportion or as cumulative positive microbiology results over the course of 6 months post-ARDS. b–i, Data show mean ± s.d., with each value representing an individual. Significant P values depicted (for P < 0.05) and obtained by Shapiro–Wilk normality test followed by a two-tailed t-test (b–i) or two-tailed Mann–Whitney U-test (c, cytoskeleton (f)). FC, fold change; gMFI, geometric mean fluorescence intensity; a.u., arbitrary units.
Low oxygen levels, a condition known as hypoxia, can do more than make us feel short of breath. According to new research, it can rewire the immune system itself, weakening the body’s long-term ability to fight infection.
The study found that when oxygen levels drop, neutrophils, a type of white blood cell that forms the body’s first line of defence, undergo genetic changes that blunt their ability to destroy invading microbes. Surprisingly, these changes don’t just affect existing cells. Hypoxia also leaves a lasting mark on bone marrow precursor cells, meaning new neutrophils produced after oxygen levels return to normal can remain impaired.
This could explain why people recovering from severe respiratory illnesses, such as acute respiratory distress syndrome (ARDS) or chronic lung disease, are often more prone to recurrent infections long after recovery.
Researchers studied neutrophils from both patients recovering from ARDS and healthy volunteers exposed to high-altitude, low-oxygen environments. They found that hypoxia alters the way DNA is organized inside these cells, changing how genes are switched on or off.
A key discovery was a process known as histone clipping, a modification to proteins that package DNA. This change can permanently alter gene expression patterns, effectively “reprogramming” the immune system to respond differently in the future.
The findings highlight how environmental stress, like oxygen deprivation, can leave a molecular “memory” in the immune system. Low oxygen doesn’t just affect breathing — it reshapes the immune system. By reprogramming how infection-fighting cells read their genetic code, hypoxia may leave the body more vulnerable to future infections, but understanding this process could pave the way for restoring immune health.
Journal article: Sanchez-Garcia, M. A., et al. 2025. Hypoxia induces histone clipping and H3K4me3 loss in neutrophil progenitors resulting in long-term impairment of neutrophil immunity. Nature Immunology.
Summary by Stefan Botha










