“Nothing in Biology Makes Sense Except in the Light of Evolution” – Theodosius Dobzhansky
Along millions of years of continuous evolution, the battle of survival between the hosts and the pathogens has been a major a determinant of natural selection. While the host develops defenses to tackle infections, the infectious agents develop and evolve to counteract these defenses and therefore survive. The current COVID-19 pandemic can also be seen in this context. Although the human body is protected by the ultimate armor of its immune system which is able to eliminate any microbe, the SARS-CoV-2 has created its own strategies to trick the human immune system.
The data on SARS-CoV-2 is still scarce. However, the evasion mechanisms of other coronaviruses such as MERS and SARS-CoV have been studied and since most of these mechanisms are conserved in most coronaviruses, they can be extrapolated to understand more about the SARS-CoV-2.
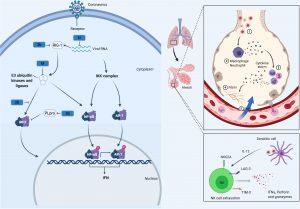
Figure 1. CoV Innate Immune Evasion. The innate immune response to CoV’s is activated upon detection of viral pathogen-associated molecular patterns, such as double-stranded RNA, via host PRRs such as RIG-I. Following viral recognition, transcription factors including NF-κB, AP-1 and IRF3 are activated and translocate to the nucleus where they induce the expression of interferons. Both MERS-CoV and SARS-CoV, through their M, N, non-structural proteins (NSP1, 3b, 4a, 4b, 5, 6), and PLpro, have developed mechanisms to interfere with these signaling pathways. This alters the cytokine secretion profile of infected cells to enhance the recruitment of myeloid immune cells over NK cells, which in turn produce more cytokines, creating a cycle of inflammation that damages the lung. Many of these processes are likely conserved in SARS-CoV-2. (Source: Taefehshokr et al., 2020)
As most of pathogens, SARS-CoV-2 can be recognized by the innate immune receptors known as pattern recognition receptors (PRRs). Normally, this recognition helps the innate immunity to eliminate the infection or to activate the adaptive immune responses, however, SARS-CoV-2 can use its proteins such as M, N, NSP1 and PLpro to interfere with these pathways. Interferons are known to be potent antiviral cytokines. In the COVID-19 patients, interferon production is greatly inhibited by these viral proteins. This less or delayed induction of IFN is able to shift the response to the production of proinflammatory cytokines.
These proinflammatory cytokines can recruit more macrophages and inflammatory cells into the lungs and hence results in leakage of vascular vessels and also impairs the adaptive immune responses. By this mechanism, the virus doesn’t just halt the immune response but rather induces exaggerated cycles of inflammation that damages the lung. This uncontrolled release of pro-inflammatory cytokines i.e. dysregulation and excessive immune responses that may cause immune damage to the body tissues is termed a cytokine storm.
Natural killer (NK) cells are also a part of the innate immunity and known to be able to eliminate the virally infected cells. It has been demonstrated that the number of activated NK cells are reduced in COVID-19 patients and they become functionally exhausted and downregulate genes necessary for tackling the virus. The mechanisms by which the SARS-CoV-2 induces these changes are still not known.
Although the SARS-CoV-2 virus primarily infect cells of the respiratory tract, it can also infect some immune cells such as monocytes, macrophages and dendritic cells; a process which can attenuate the immune cells functions. These cells are known to be Antigen-Presenting cells (APCs) and are capable of presenting the viral antigens to the lymphocytes in the lymph nodes to trigger an adaptive immune response. However, it was found that SARS-CoV-2 can downregulate major histocompatibility complex (MHC) molecules which are necessary for this presentation. The virus also limits the dendritic cells maturation and renders them incapable of activating the adaptive immune cells.
Adaptive immunity is specific and long-lasting and is the major player in fighting viral infections. This arm of the immune system needs an activation by the innate immune cells which lost their functions due to SARS-CoV-2 infection. Not only this, but also the cytokine storm is hypothesized to induce the clinically observed lymphopenia in COVID-19 patients. Other hypotheses of how the SARS-CoV-2 affects the adaptive immunity include that the infection can result in exhaustion of T cells, where they can’t perform their functions anymore and also that the infection can interfere with the expansion of T cells through downregulating some genes necessary for the lymphocytes proliferation.
The mechanisms by which SARS-CoV-2 manipulated the immune system are not so well understood yet. The great inter-individual variability of the immune response and why some individuals can control the infection while others can’t is still not clear. Further studies are still needed to understand the interaction between the virus, the environment and the host. Understanding such mechanisms will help us better understand how our immune systems function and can be considerable points in developing novel immunotherapies and vaccines against COVID-19.
References:
- Kumar, S., Nyodu, R., Maurya, V. K., & Saxena, S. K. (2020). Host Immune Response and Immunobiology of Human SARS-CoV-2 Infection. Coronavirus Disease 2019 (COVID-19): Epidemiology, Pathogenesis, Diagnosis, and Therapeutics.
- Taefehshokr, N., Taefehshokr, S., Hemmat, N., & Heit, B. (2020). Covid-19: Perspectives on Innate Immune Evasion. Frontiers in immunology.
- Tavakolpour, S., Rakhshandehroo, T., Wei, E. X., & Rashidian, M. (2020). Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunology letters.
- Ye, Q., Wang, B., & Mao, J. (2020). The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. The Journal of infection.
Article by Hatem Abouguendia










